BT
Metal Indicator
-
Product codeB015 BT
-
CAS No.1787-61-7
-
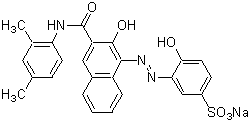
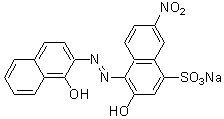
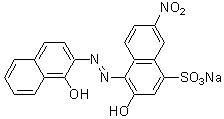
Chemical name2-Hydroxy-1-(1-hydroxy-2-naphthylazo)-6-nitro-4-naphthalenesulfonic acid, sodium salt
-
MWC20H12N3NaO7S=461.38
| Unit size | Price | Item Code |
|---|---|---|
| 25 g | Find your distributors | B015-10 |
Product Description
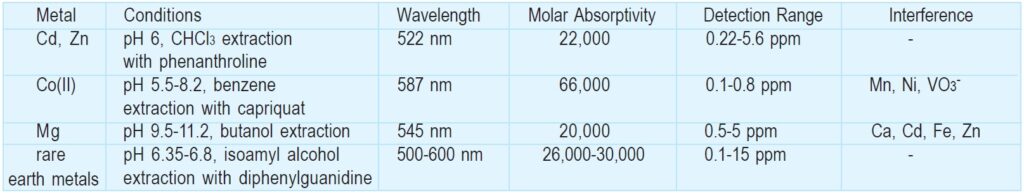
BT in aqueous solution changes color according to pH: red at pH<6, blue at pH 7-11, and orange at pH>12. Its proton dissociation constants are reported to be pKa2=6.3 and pKa3=11.55 (m=0.08, 20ºC). BT is one of the most commonly used indicators of Ca, Mg, Zn, Cd, Pb, Hg, and rare earth metals for EDTA chelate titration. BT solution turns red in the presence of the metals at pH 10, and then changes to blue at the endpoint of the titration. Cu and Fe(III), as well as Al, Co, and Mn(III), should be masked with KCN because they interfere with the color change. Triethanolamine is also a suitable masking reagent for Al, Fe(III), and Mn(III). Since metal ions such as Mn(III) and Fe(III) oxidize BT, hydroxylamine and hydrochloride should be added to prevent oxidation.
Chemical Structure
Technical info
References
1) K. Ueno, T. Imamura and K. L. Cheng, "Handbook of Organic Analitical Reagents 2nd Edition", CRC Press, 1992.
2) P. F. Lott, K. L. Cheng and C. H. Kwan, "Spectrophotometric Determination of Thorium with Eriochrome Black T", Anal. Chem., 1960, 32, 1702.
Handling and storage condition
| Appearance: | Black to blackish brown powder |
|---|---|
| Solubility in water: | To pass test (clear, dark reddish purple) |
| Solubility in Methyl alcohol: | To pass test (almost clear, dark reddish purple) |
| Absorbance in methyl alcohol: | ≧ 0.250 (around 520 nm) |
| Absorbance in borate buffer: | ≧ 0.400 (around 620 nm) |
| Loss on drying (105°C): | ≦ 7.0 % |
| Sensitivity: | To pass test |
| IR spectrum: | Authentic |
| Ambient temperature |