Previous Science Note
| Cellular senescence spreads through tissues via secreted factors, contributing to tissue dysfunction, chronic inflammation, and impaired regeneration. This Science Note introduces recent insights into the in vivo spread of senescence and highlights emerging evidence that rejuvenating factors can also spread, suggesting new avenues for modulating aging and restoring tissue function. | ||||||||||||||||||||
|
Mitochondrial DNA released by senescent tumor cells enhances PMN-MDSC-driven immunosuppression through the cGAS-STING pathway (Immunity, 2025) Highlighted technique: To assess mtDNA transfer from tumour senescent cells, extracellular vesicles (EVs) were isolated and confirmed to be mtDNA enriched. Their uptake by BM-MDSCs led to intracellular mtDNA accumulation, whereas EV-suppressed senescent cells failed to induce mtDNA accumulation during co-culture with BM-MDSCs. Related technique Cellular Senescence Detection, Exosome Labeling Dye |
||||||||||||||||||||
|
Hepatocellular senescence induces multi-organ senescence and dysfunction via TGFβ (Nature Cell Biology, 2024) Related technique Cellular Senescence Detection |
||||||||||||||||||||
|
Small extracellular vesicles from young plasma reverse age-related functional declines by improving mitochondrial energy metabolism (Nature Aging, 2024) Highlighted technique: In this study, researchers collected sEVs from the blood plasma of young mice and injected them into older mice to observe the effects. They examined lifespan, cognitive function and mitochondrial activity, which is often impaired in ageing, to show the reversal of signs of ageing in the mice. Related technique Cellular Senescence Detection, Mitochondrial membrane potential detection |
||||||||||||||||||||
Related Techniques (click to open/close)
|
||||||||||||||||||||
Application Note I (click to open/close)
|
||||||||||||||||||||
|
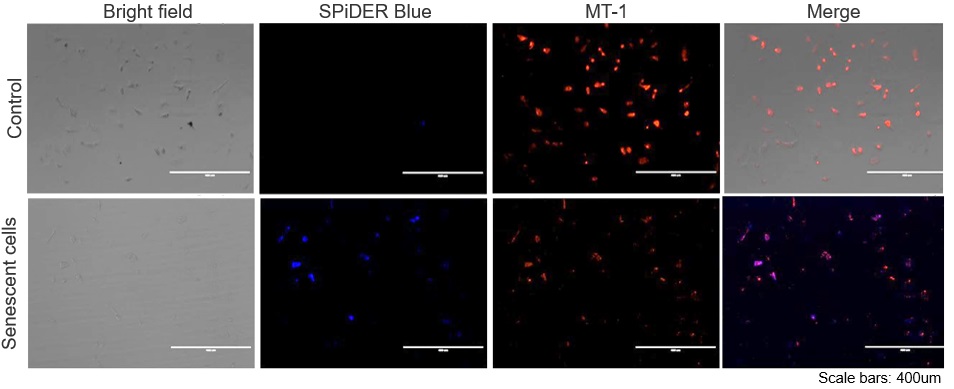
The senescent cell detection dye SPiDER Blue (SG07) and the mitochondrial membrane potential (MMP) dye MT-1 (MT13) were used to stain human microglial cells. Microscopy revealed that, compared to control cells, senescence-induced cells showed reduced MMP and increased SPiDER Blue fluorescence, reflecting elevated SA-β-Gal activity. *This data was kindly provided by Dr. Supriya D. Mahajan, Department of Medicine, Jacobs School of Medicine & Biomedical Sciences. 1. Seed human microglia cells into a dish and incubate in an incubator set at 37 ℃ and equilibrated with 95% air and 5% CO2. |
Application Note II (click to open/close)
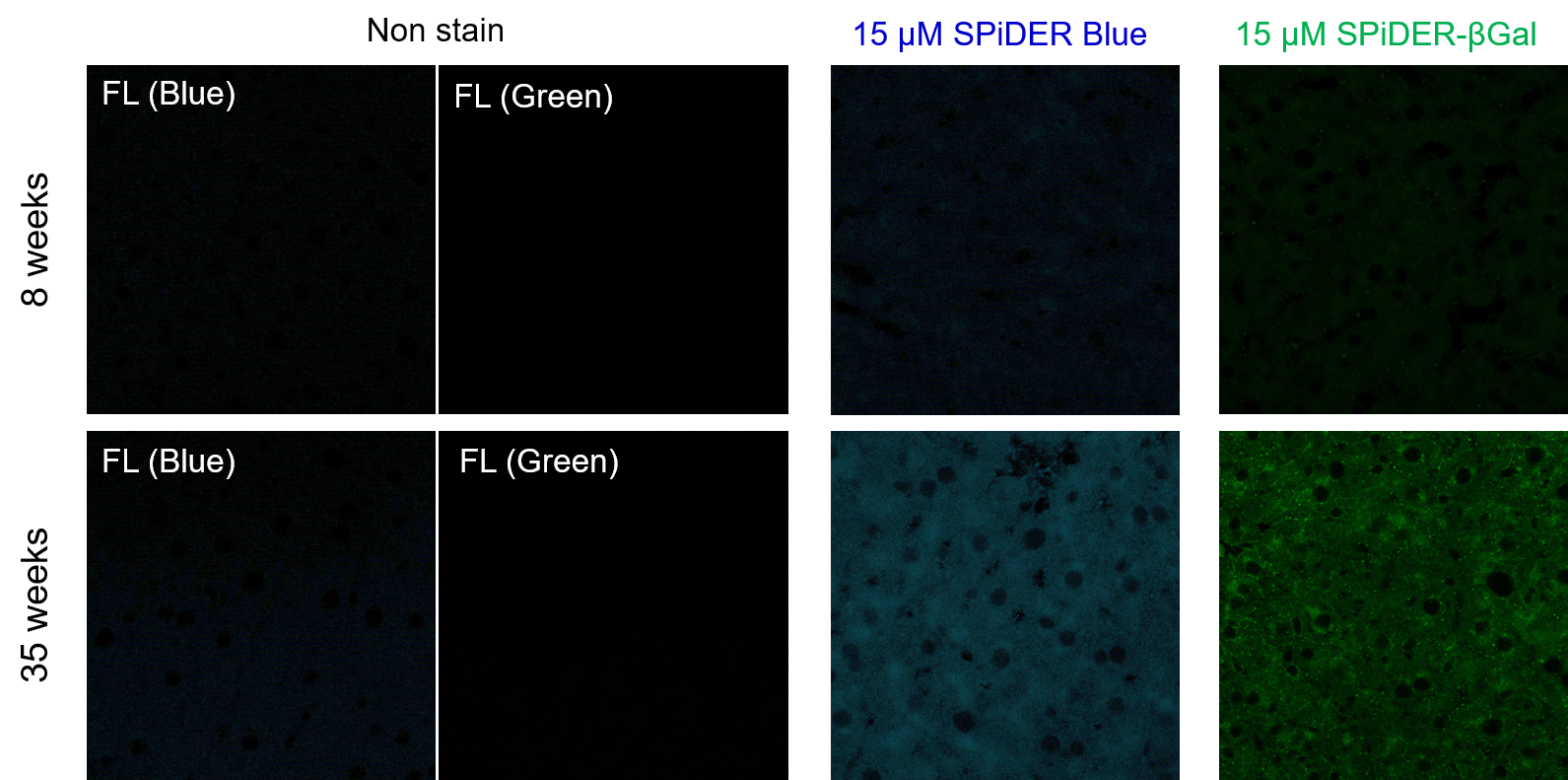
> Increased Senescence in Aged Adipose Tissue
|
Frozen liver adipose tissue sections from 8-week-old and 35-week-old mice were stained with senescence detection probes SPiDER Blue (SG07) and SPiDER-βGal (SG02). Confocal microscopy revealed a marked increase in fluorescence intensity only in the 35-week-old samples, indicating an age-associated accumulation of senescent cells in older tissue. 1. 8-week-old and 35-week-old mouse liver adipose tissue (frozen sections) samples were prepared on glass slides. [Detection conditions] |