Previous Science Note
| Mitochondria under stress activate distinct recovery pathways involving lipid mobilization, organelle contact sites, and cytoskeletal dynamics. This Science Note highlights recent studies revealing how cells coordinate lipid metabolism, mitochondria–ER connections, and actin polymerization to restore mitochondrial structure and function. | ||||||||||||||||||||||||||
|
Triacylglycerol mobilization underpins mitochondrial stress recovery (Nature Cell Biology, 2025) Highlighted technique: Researchers labeled TAG stores in yeast with carbon-labeled oleic acid to track fat mobilization after mitochondrial stress. This helped show that fatty acids from TAG, released by Tgl3-5p lipases, are used to make cardiolipin during mitochondrial recovery. Related technique Lipid Droplet Detection, OCR Measurement |
||||||||||||||||||||||||||
|
ER-mitochondria contacts mediate lipid radical transfer via RMDN3/PTPIP51 phosphorylation to reduce mitochondrial oxidative stress (Nature Communications, 2025) Highlighted technique: NanoBiT is a split-luciferase system that emits light when two protein fragments come close, allowing real-time tracking of molecular interactions in living cells. In this study, the authors created MERBiT by tagging mitochondrial TOMM20 with SmBiT and ER-localized Sec61β with LgBiT in HeLa cells, enabling quantitative monitoring of reversible MERCs during mitochondrial stress. Related technique Mitochondrial Staining, Mitochondrial Lipid Peroxide Detection (used in this article) |
||||||||||||||||||||||||||
|
Summary: This study reveals that actin polymerization is essential for mitochondrial fusion, uncovering a new role in organelle dynamics. It shows that fusion requires Arp2/3 (a complex that builds branched actin), INF2 (a protein that drives actin filament growth), and that actin appears at fusion sites before MFN2 (a key fusion protein), suggesting actin actively guides the fusion process. Highlighted technique: In this study, PA-GFP (photoactivatable GFP), a protein that remains non-fluorescent until exposed to 405 nm light, was used to label specific regions of the mitochondrial network. This allowed the authors to track fluorescence spread through fused mitochondria and quantitatively demonstrate that mitochondrial fusion depends on actin polymerization. Related technique Mitochondrial Membrane Potential Detection |
||||||||||||||||||||||||||
Related Techniques (click to open/close)
|
||||||||||||||||||||||||||
Application Note I (click to open/close)
|
||||||||||||||||||||||||||
|
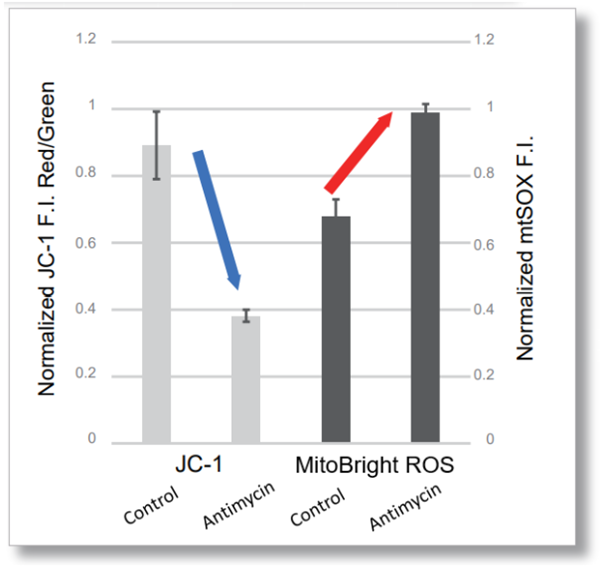
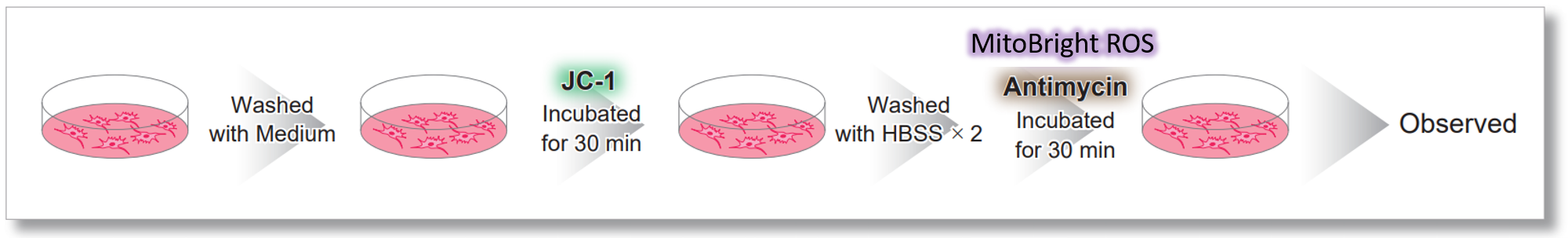
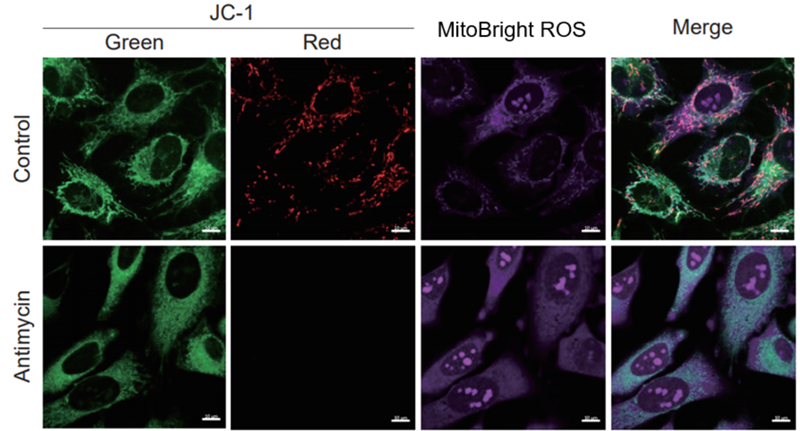
After HeLa cells were washed with HBSS, co-stained with MitoBright ROS Deep Red and mitochondrial membrane potential staining dye (JC-1: code MT09), and the generated mitochondrial ROS and membrane potential were observed simultaneously. As a result, the decrease in mitochondrial membrane potential and the generation of mitochondrial ROS are simultaneously observed. |
|
|
|
|




 <Imaging Conditions>(Confocal microscopy)
<Imaging Conditions>(Confocal microscopy)