|
Neuronal lipids support mitochondrial energy production and membrane phospholipid composition, both important for neuronal function. Glucose has been positioned as the major energy source in neurons, yet recent work shows that triglycerides stored in presynaptic lipid droplets can serve as fuel under low glucose, and that inhibiting this utilization causes rapid hypothermia in mice. Because polyunsaturated fatty acids (PUFAs) can contribute to ferroptosis, PUFA lowering is sometimes discussed as protective, but an inherited ALS/FTD form shows reduced PUFA in neuronal membrane phospholipids, and boosting fatty acid desaturation to restore PUFA production improves neurodegeneration-related readouts. Together, these findings indicate that neuronal lipid utilization and membrane lipid composition can influence disease-relevant readouts. |
||||||||||||||||||||||
|
Triglycerides are an important fuel reserve for synapse function in the brain (Nature Metabolism, 2025) Highlighted technique: To track the transfer of lipid droplet–associated fatty acids to mitochondria in cultured neurons, the authors performed a fluorescent fatty-acid tracer assay. Neurons were loaded with the fatty-acid analog BODIPY 558/568 C12 (Red-C12) under conditions that inhibit lipid droplet triglyceride breakdown, and mitochondria and lipid droplets were co-stained and imaged by confocal microscopy to confirm and quantify Red-C12 co-localization with mitochondria. |
||||||||||||||||||||||
|
Neuronal polyunsaturated fatty acids are protective in ALS/FTD (Nature Neuroscience, 2025) Highlighted technique: To test whether increasing neuronal PUFA levels can rescue a disease-relevant cellular phenotype, the authors generated human spinal neurons from iPS cells derived from patients with a major inherited form of ALS/FTD and non-neurological controls, and boosted intracellular PUFA production by expressing fatty-acid desaturases. They then applied a defined glutamate excitotoxic stress and quantified phenotype changes by measuring cell death with a fluorescent dead-cell stain and cell survival using a standard viability assay. |
||||||||||||||||||||||
Metabolic Activity Assays (click to open/close)
|
||||||||||||||||||||||
Application Note (click to open/close)
|
||||||||||||||||||||||
|
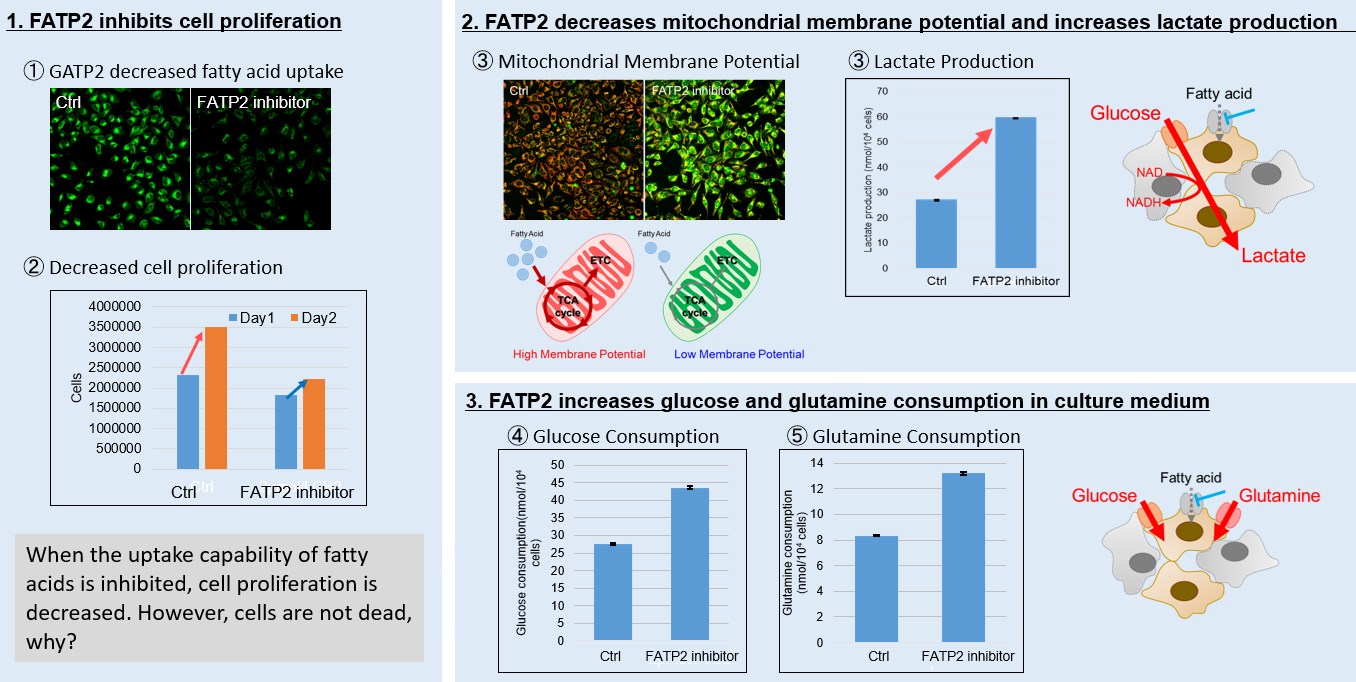
Image 1: Inhibition of fatty acid uptake results in reduced cell proliferation, though it does not lead to cell death. This was determined through the use of a Cell Counting Kit-8 and Fatty Acid Uptake Kit. Image 2: Fatty acid starvation shifts cellular metabolism from OXPHOS to glycolysis, as indicated by the Glycolysis/JC-1 MitoMP Assay Kit. Image 3: When fatty acid uptake is inhibited, a compensatory increase in glucose and glutamine uptake occurs to preserve cell viability, as observed using the Glucose Assay Kit and Glutamine Assay Kit. Products in Use for Cell Proliferation/Cytotoxicity Assay for Glycolysis Assay and Mitochondrial Membrane Potential Detection for Glucose and Glutamine Consumption Assay |