|
Recent research on cancer progression shows that mtDNA mutations affect cellular metabolism and promote tumor growth. Here are some of the studies showing how mtDNA mutations drive metabolic changes and have implications for cancer therapy.
Mitochondrial DNA (mtDNA) plays a key role in cancer progression by influencing cellular metabolism and oxidative phosphorylation. Mutations in mtDNA can lead to metabolic shifts, such as the Warburg effect, that promote tumor growth and alter the tumor microenvironment. These mtDNA alterations can increase the production of reactive oxygen species (ROS), which drive pro-tumor signaling pathways such as TGFβ/Smad. In addition, mtDNA mutations and their transfer via extracellular vesicles (EVs) can affect tumor-stroma communication and potentially serve as biomarkers and therapeutic targets in cancer treatment.
|
-
Mitochondrial DNA mutations drive aerobic glycolysis to enhance checkpoint blockade response in melanoma
Click here for the original article: Mahnoor Mahmood, et. al., Nature Cancer, 2024.
Point of Interest
- DNA mutations in tumors promote metabolic shifts and anti-tumor immune responses, sensitizing them to checkpoint blockade therapies.
- Engineered mtDNA mutations in melanoma reshaped the tumor microenvironment, reducing neutrophils and enhancing immune responses in mice and humans.
- Tumors with >50% mtDNA mutations showed a 2.5-fold improved response to checkpoint blockade, highlighting the therapeutic potential of mtDNA.
-
Mitochondrial genome transfer drives metabolic reprogramming in adjacent colonic epithelial cells promoting TGFβ1-mediated tumor progression
Click here for the original article: Bingjie Guan, et. al., Nature Communications, 2024.
Point of Interest
- Tumor-derived EV-mtDNA enhances mitochondrial respiration, ROS production and TGFβ1 expression, promoting colon cancer progression.
- EV-mtDNA from cancer cells activates ROS-driven RelA translocation in neighboring cells, regulating TGFβ1 and promoting tumor growth.
- EV-mtDNA serves as a potential biomarker and therapeutic target for paracrine metabolic communication in colon cancer.
-
Plasma cell–derived mtDAMPs activate the macrophage STING pathway, promoting myeloma progression
Click here for the original article: Aisha Jibril, et. al., Blood, 2024.
Point of Interest
- Elevated cell-free mtDNA in multiple myeloma activates macrophages via the STING pathway and promotes disease progression in the bone marrow.
- Multiple myeloma (MM) cell-derived mtDAMPs induce chemokine upregulation in bone marrow macrophages, contributing to MM cell retention and tumor growth.
- Inhibition of STING signaling or chemokine response reduces multiple myeloma tumor burden and induces MM cell egress from the bone marrow.
|
| Related Techniques |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
- Exosome Membrane Fluorescent Staining
- ExoSparkler Exosome Membrane Labeling Kit-Green/ Red/ Deep Red
|
|
|
| Related Applications |
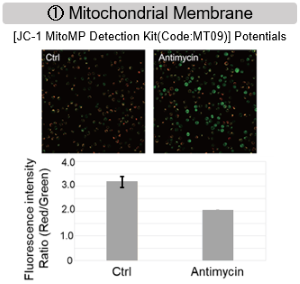
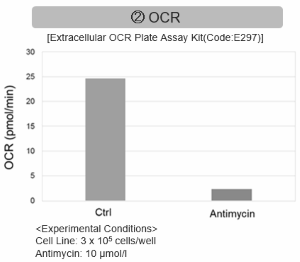
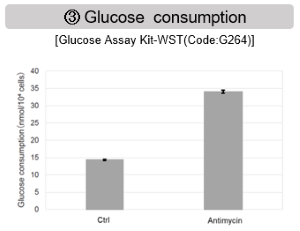
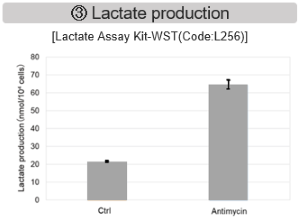
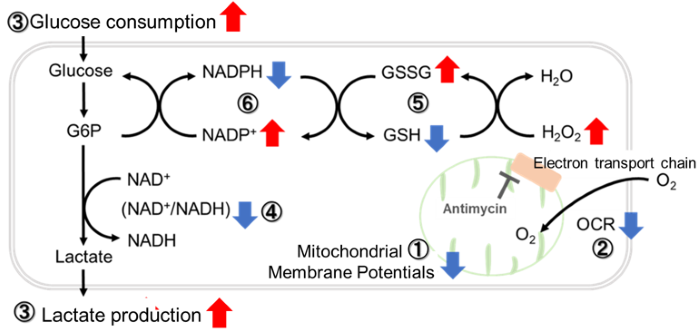
Inhibition of Mitochondrial Electron Transport Chain
-
-
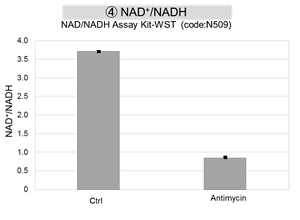
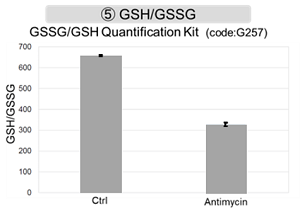
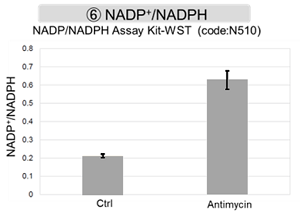
Antimycin stimulation of Jurkat cells was used to evaluate the changes in cellular state upon inhibition of the mitochondrial electron transport chain using a variety of indicators.
The results showed that inhibition of the electron transport chain resulted in (1) a decrease in mitochondrial membrane potential and (2) a decrease in OCR. In addition, (3) the NAD+/NADH ratio of the entire glycolytic pathway decreased due to increased metabolism of pyruvate to lactate to maintain the glycolytic pathway, (4) GSH depletion due to increased reactive oxygen species (ROS), and (6) increase in the NADP+/NADPH ratio due to decreased NADH required for glutathione biosynthesis were observed.
|
Metabolic Pathway Dependence Across Cell Lines
Evaluation by Lactate production and ATP levels
-
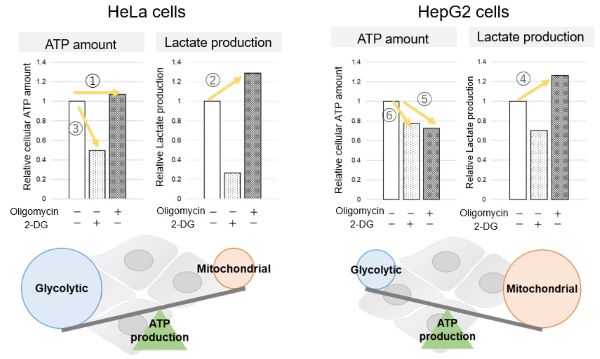
-
<Evaluation by Lactate production and ATP levels>
We confirmed the changes in ATP and Lactate production when ATP synthesis by OXPHOS was inhibited by Oligomycin stimulation and by 2-Deoxy-D-glucose (2-DG) in the glycolytic pathway. The results showed that HeLa cells depend on Glycolysis and HepG2 cells depend on OXPHOS to synthesize ATP.
When OXPHOS was inhibited in HeLa cells, ATP levels remained unchanged (①), and lactate production increased (②). This suggests that even when OXPHOS is inhibited, glycolysis can be further activated. Conversely, when glycolysis is inhibited, ATP levels decrease significantly (③), indicating that energy production depends on glycolysis. On the other hand, when OXPHOS was inhibited in HepG2 cells, lactate production increased (④), indicating that the cells attempt to compensate for energy production by enhancing glycolysis, but ATP levels still decrease (⑤). This means that even with increased glycolysis, ATP production is not sufficiently compensated. Furthermore, ATP levels decrease more when glycolysis is inhibited (⑥), suggesting that energy production in HepG2 cells depends more on OXPHOS than glycolysis.
Products in Use
- Glycolysis/OXPHOS Assay Kit
Evaluation by OCR value
-
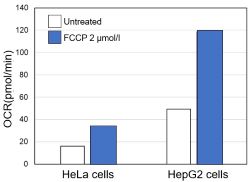 
-
<Evaluation by OCR value>
Using the same number of cells, we measured the OCR value when cellular oxygen consumption was promoted by stimulating the cells with FCCP, a mitochondrial uncoupling agent. The results showed that HepG2 cells had higher OCR values than HeLa cells, suggesting a greater dependence on OXPHOS, correlating with the results obtained from ATP level and Lactate production.
Products in Use
- Extracellular OCR Plate Assay Kit
|