|
Mitochondrial activity and transfer are increasingly recognized for their roles in cancer progression and therapy. Tumor cells can enhance their survival and resistance to therapy by acquiring mitochondria from surrounding stromal cells through a process known as mitochondrial transfer. This transfer not only helps cancer cells meet their increased energy and metabolic demands but also aids in evading apoptosis induced by therapeutic agents. As a result, targeting the mechanisms of mitochondrial transfer and disrupting these interactions is being explored as a novel therapeutic strategy to enhance the effectiveness of cancer treatments and reduce resistance.
|
-
Mitochondrial metabolism sustains CD8+ T cell migration for an efficient infiltration into solid tumors
Click here for the original article: Luca Simula, et. al., Nature Communications, 2024.
Point of Interest
- Mitochondrial oxidation of glucose and glutamine, but not fatty acids, is required for interstitial motility of CD8+ T cells.
- For T cells to migrate, both ATP and ROS produced by mitochondria are required.
- Increasing mitochondrial activity improves intratumoral migration of CD8+ T cells and recruitment of CAR T cells to tumor islets.
-
Cancer cells reprogram to metastatic state through the acquisition of platelet mitochondria
Click here for the original article: Wenkan Zhang, et. al., Cell Reports, 2023.
Point of Interest
- Cancer cells acquire platelet mitochondria via the PINK1/Parkin-Mfn2 pathway to be reprogrammed to a metastatic state.
- Transferred platelet mitochondria promoted glycolytic metabolism and increased glutathione peroxidase expression.
- GSH eliminates ROS and increase, which leads to increase GSSG levels and ultimately to enhance lung metastasis of osteosarcoma in the presence of platelet mitochondria.
-
Defects of mitochondria-lysosomes communication induce secretion of mitochondria-derived vesicles and drive chemoresistance in ovarian cancer cells
Click here for the original article: Sinforosa Gagliardi, et. al., Cell Commun Signal., 2024.
Point of Interest
- Cisplatin chemoresistant cells are characterized by impaired late endocytic trafficking and increased secretion of RAB7-positive mitochondria-derived vesicles (MDVs).
- MDVs can be secreted by cisplatin chemoresistant cells and deliver cisplatin outside the cells.
- MDVs purified from chemoresistant cells induce chemoresistance in recipient cells via a RAB7-modulated process.
- The MDVs localize to mitochondria and slow down mitochondrial activity, leading to mitochondrial dysfunction, lysosomal deficit, and ultimately increased MDVs.
|
| Related Techniques |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Related Applications |
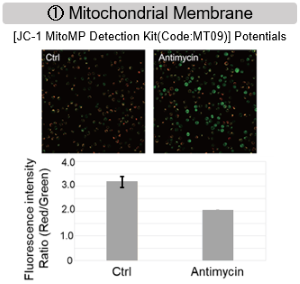
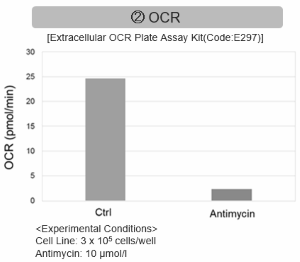
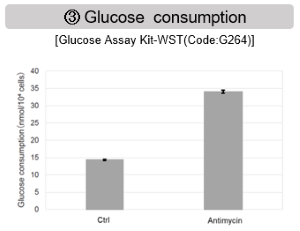
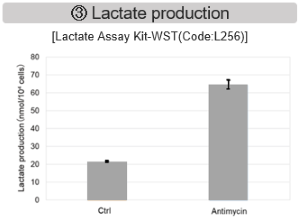
Inhibition of Mitochondrial Electron Transport Chain
-
-
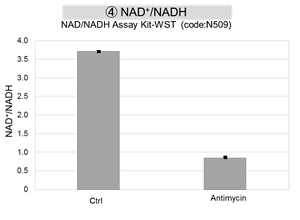
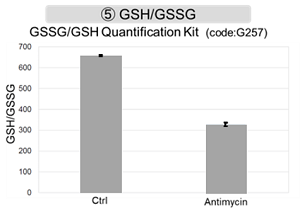
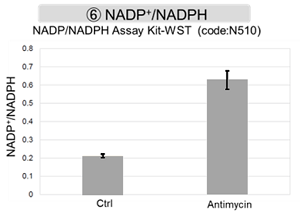
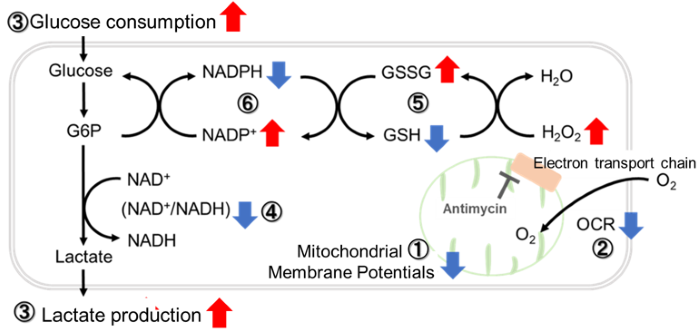
Antimycin stimulation of Jurkat cells was used to evaluate the changes in cellular state upon inhibition of the mitochondrial electron transport chain using a variety of indicators.
The results showed that inhibition of the electron transport chain resulted in (1) a decrease in mitochondrial membrane potential and (2) a decrease in OCR. In addition, (3) the NAD+/NADH ratio of the entire glycolytic pathway decreased due to increased metabolism of pyruvate to lactate to maintain the glycolytic pathway, (4) GSH depletion due to increased reactive oxygen species (ROS), and (6) increase in the NADP+/NADPH ratio due to decreased NADH required for glutathione biosynthesis were observed.
|
Metabolic Pathway Dependence Across Cell Lines
Evaluation by Lactate production and ATP levels
-
-
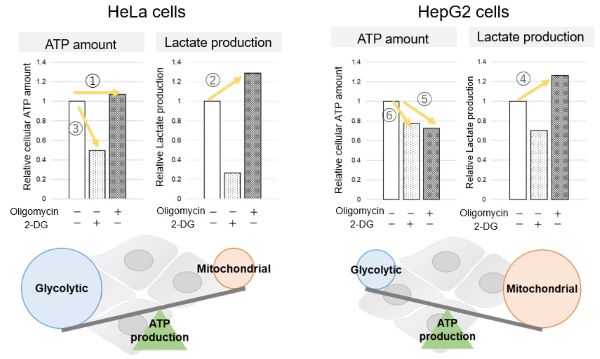
<Evaluation by Lactate production and ATP levels>
We confirmed the changes in ATP and Lactate production when ATP synthesis by OXPHOS was inhibited by Oligomycin stimulation and by 2-Deoxy-D-glucose (2-DG) in the glycolytic pathway. The results showed that HeLa cells depend on Glycolysis and HepG2 cells depend on OXPHOS to synthesize ATP.
When OXPHOS was inhibited in HeLa cells, ATP levels remained unchanged (①), and lactate production increased (②). This suggests that even when OXPHOS is inhibited, glycolysis can be further activated. Conversely, when glycolysis is inhibited, ATP levels decrease significantly (③), indicating that energy production depends on glycolysis. On the other hand, when OXPHOS was inhibited in HepG2 cells, lactate production increased (④), indicating that the cells attempt to compensate for energy production by enhancing glycolysis, but ATP levels still decrease (⑤). This means that even with increased glycolysis, ATP production is not sufficiently compensated. Furthermore, ATP levels decrease more when glycolysis is inhibited (⑥), suggesting that energy production in HepG2 cells depends more on OXPHOS than glycolysis.
Products in Use
- Glycolysis/OXPHOS Assay Kit
Evaluation by OCR value
-
-
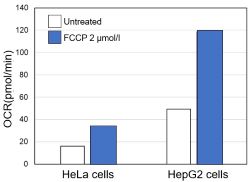
<Evaluation by OCR value>
Using the same number of cells, we measured the OCR value when cellular oxygen consumption was promoted by stimulating the cells with FCCP, a mitochondrial uncoupling agent. The results showed that HepG2 cells had higher OCR values than HeLa cells, suggesting a greater dependence on OXPHOS, correlating with the results obtained from ATP level and Lactate production.
Products in Use
- Extracellular OCR Plate Assay Kit
|