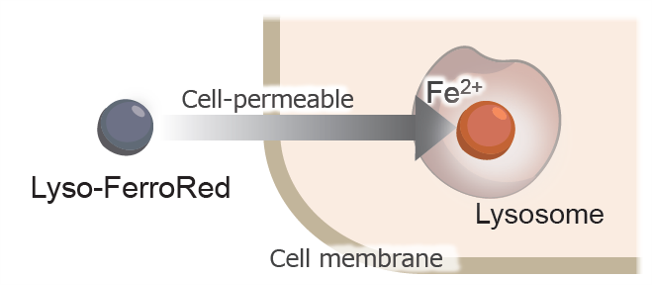
Lyso-FerroRed

Lysosomal Iron Ion Detection Reagent
- Detects Fe²⁺ within the lysosomes of living cells
- Enables imaging via fluorescence microscopy and quantification with plate readers and flow cytometers
-
Product codeL270 Lyso-FerroRed
-
CAS No.1616953-95-7
-
Chemical name3'-(dimethylamino)-N,N-dimethyl-3H-spiro[isobenzofuran-1,9'-xanthen]-6'-amine oxide
| Unit size | Price | Item Code |
|---|---|---|
| 35 nmol | $380.00 | L270-10 |
*35 nmol can be used for 17 assays at 35 mm dish (final concentration 1 µmol/l).
The importance of Lysosomal Iron in Ferroptosis
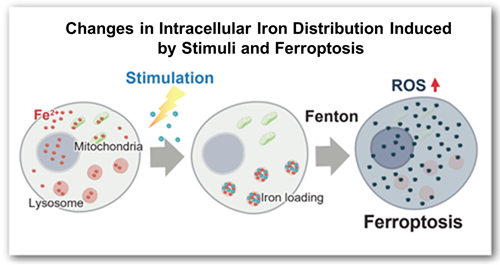
Ferroptosis, a type of cell death induced by iron-dependent lipid peroxidation, has been established as a factor in various diseases, including cancer, neurodegenerative diseases, and age-related disorders. While previous research primarily focused on total cellular iron content, recent studies have emphasized the importance of iron localization within organelles, particularly lysosomes. Lysosomes are known not only as sites responsible for cellular degradation and recycling, but also as storage sites for iron. Some researchers have suggested that iron dynamics within lysosomes may trigger ferroptosis*. Against this backdrop, techniques for selectively detecting and evaluating lysosomal iron have garnered significant attention.
* Y. Saimoto, et al., Nature Communications, 2025, 16, 3554.
Ferroptosis Analysis Products
| Product Name | Target | Ditection Properties |
|---|---|---|
| FerroOrange | Intracellular Fe2+ | Microscopy, Plate reader Ex: 543 nm / Em: 580 nm |
| Mito-FerroGreen | Mitochondrial Fe2+ | Microscopy Ex: 505 nm / Em: 535 nm |
| Lyso-FerroRed | Lysosomal Fe2+ | Microscopy, FCM, Plate reader Ex: 551 nm / Em: 571 nm |
| Iron Assay Kit -Colorimetric- | Fe2+ and Fe3+ | Plate reader Colorimetric, λ: 593 nm |
| Liperfluo | Lipid Peroxide | Microscopy, FCM Ex: 488 nm / Em: 500-550 nm |
| Lipid Peroxidation Probe -BDP 581/591 C11- |
Lipid Peroxidation Process |
Microscopy, FCM, Plate reader |
| MDA Assay Kit | Malondialdehyde | Plate reader Fluorescence, Ex: 540 nm / Em: 590 nm Colorimetric, λ: 532 nm |
| Cystine Uptake Assay Kit | Cystine uptake | Plate reader Ex: 485 nm / Em: 535 nm |
| GSSG/GSH Quantification Kit | GSSG and GSH | Plate reader Colorimetric, λ: 405 nm |
Manual
Technical info
More than 10 years have passed since Stockwell et al. discovered ferroptosis, which is caused by an iron ion-dependent accumulation of lipid peroxides. It has become clear that ferroptosis is a form of cell death, in which free iron (Fe2+) generates reactive oxygen species (ROS) through the Fenton reaction1), which in turn oxidize lipids. More recently, it has been reported that lipid oxidation damages lysosomal membranes and causes iron to leak from lysosomes, thereby spreading it to other cellular organelles2). Lyso-FerroRed can detect intracellular lysosomal free iron (Fe2+). Lyso-FerroRed penetrates the cell membrane and selectively reacts with Fe2+ in lysosomes, emitting strong fluorescence. Reaction between Lyso-FerroRed and Fe2+ is irreversible and differs from those based on the detection principle of calcium iron probes such as Fluo 3. Lyso-FerroRed has no chelating ability.
1) S. J. Dixon, et al., Cell, 2012, 149, 1060–1072
2) Y. Saimoto, et al., Nature Communications, 2025, 16, 3554
This product was developed under the guidance of Professor Tasuku Hirayama of Gifu Pharmaceutical University.
Selection of Iron Detection Reagents
Select the appropriate reagent based on your experimental method and detecting equipment.
| Lyso-FerroRed | FerroOrange | Mito-FerroGreen | Iron Assay Kit -Colorimetric- | |
|
Intracellular localization |
Lysosome |
Intracellular |
Mitochondria |
ー (Tissue) |
|---|---|---|---|---|
|
Detection properties |
Fluorometric |
Fluorometric |
Fluorometric |
Colorimetric |
|
Supported |
Microscope, Plate reader, |
Microscope, Plate reader(Cy3) |
Microscope(FITC, GFP) |
Plate reader |
|
Target |
Lysosomal Fe2+ in live cells |
Intracellular Fe2+ in live cells |
Mitochondrial Fe2+ in live cells |
Fe2+ and Fe3+ in tissue |
|
Usage count |
35 nmol can be used for 17 assays at 35 mm dish |
1 tube (24 µg) can be used for 17 assays at 35 mm dish |
1 set (50 µg x 2) can be used for 10 assays at 35 mm dish |
50 tests |
Detection and Quantification of Lysosomal Fe²⁺
We confirmed changes in intracellular Fe²⁺ within lysosomes of HT-1080 cells using Lyso-FerroRed. We achieved this by varying the presence or absence of the iron-chelating reagent Bpy (final concentration: 1 mmol/l) and by adding iron (ferrous ammonium sulfate, final concentration: 100 μmol/l).
Fluorescence microscopy imaging
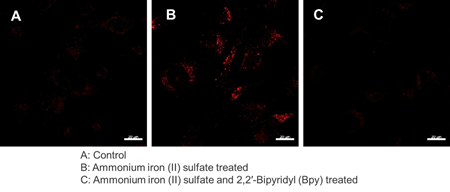
[Detection condition] Ex/Em = 564 nm / 565-620 nm
Quantification by plate assay
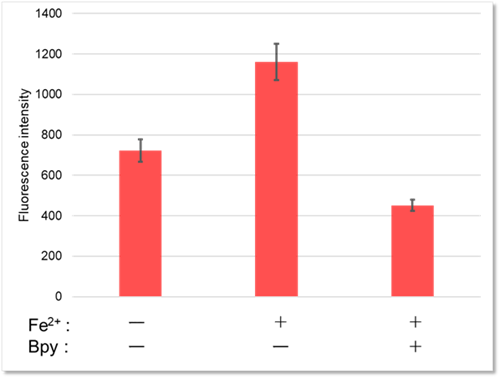
[Detection condition] Ex/Em = 550 nm / 580 nm
Detection by flow cytometry
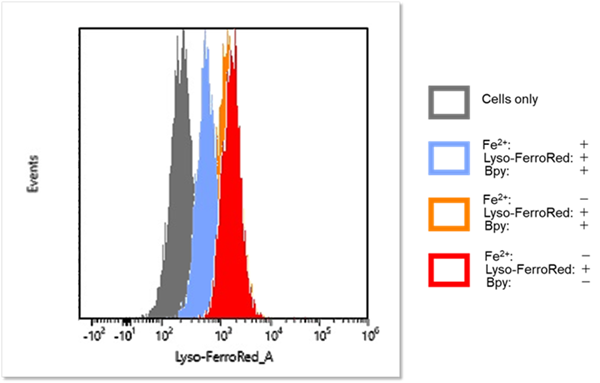
[Detection condition] Ex/Em = 565 nm / 565-617 nm
High Selectivity for Fe2+
One milliliter of 50 mmol/l HEPES buffer (pH 7.4) was mixed with 2 µl of 1 mmol/l Lyso-FerroRed and various metals. After reacting at room temperature for one hour, the fluorescence intensity was measured.
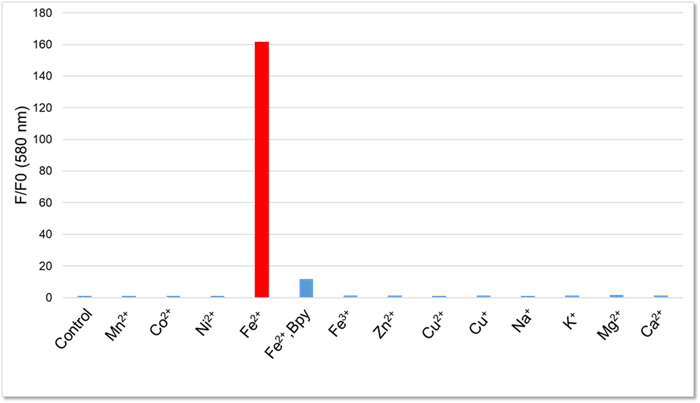
[Final concentration]
Lyso-FerroRed:2 μmol/l
Mn, Co, Ni, Fe2+, Fe3+, Zn, Cu : 20 nmol/l
Na, K, Mg, Ca : 1 mmol/l
Bpy : 100 µmol/l
[Detection conditions]
Ex: 550 nm, Em: 580 nm
Definite Lysosomal Localization
To confirm the lysosomal localization of Lyso-FerroRed, we performed co-staining with various organelle-staining reagents and confirmed that Lyso-FerroRed selectively stains lysosomes.
Lysosome staining: LysoPrime Deep Red (Product Code: L264)
Lipid droplet staining: Lipi-Deep Red (product code LD04)
Mitochondria staining: MitoBright LT Deep Red (product code MT12)
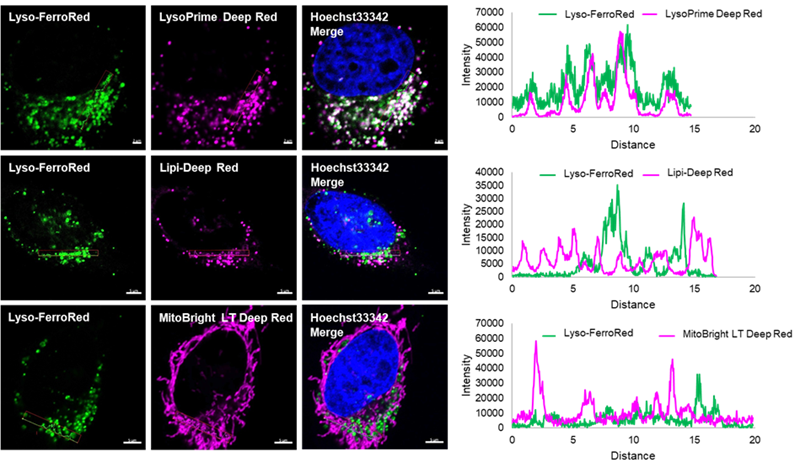
*Lyso-FerroRed is displayed in pseudo-color to make it easier to visualize the merged images.
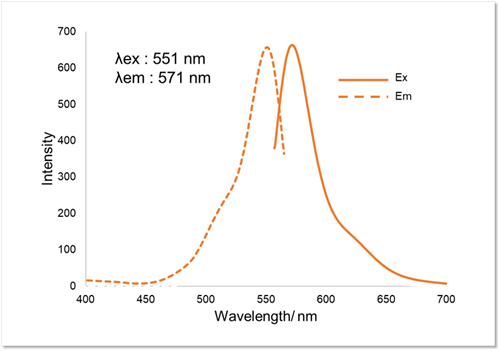
Fluorescence Property
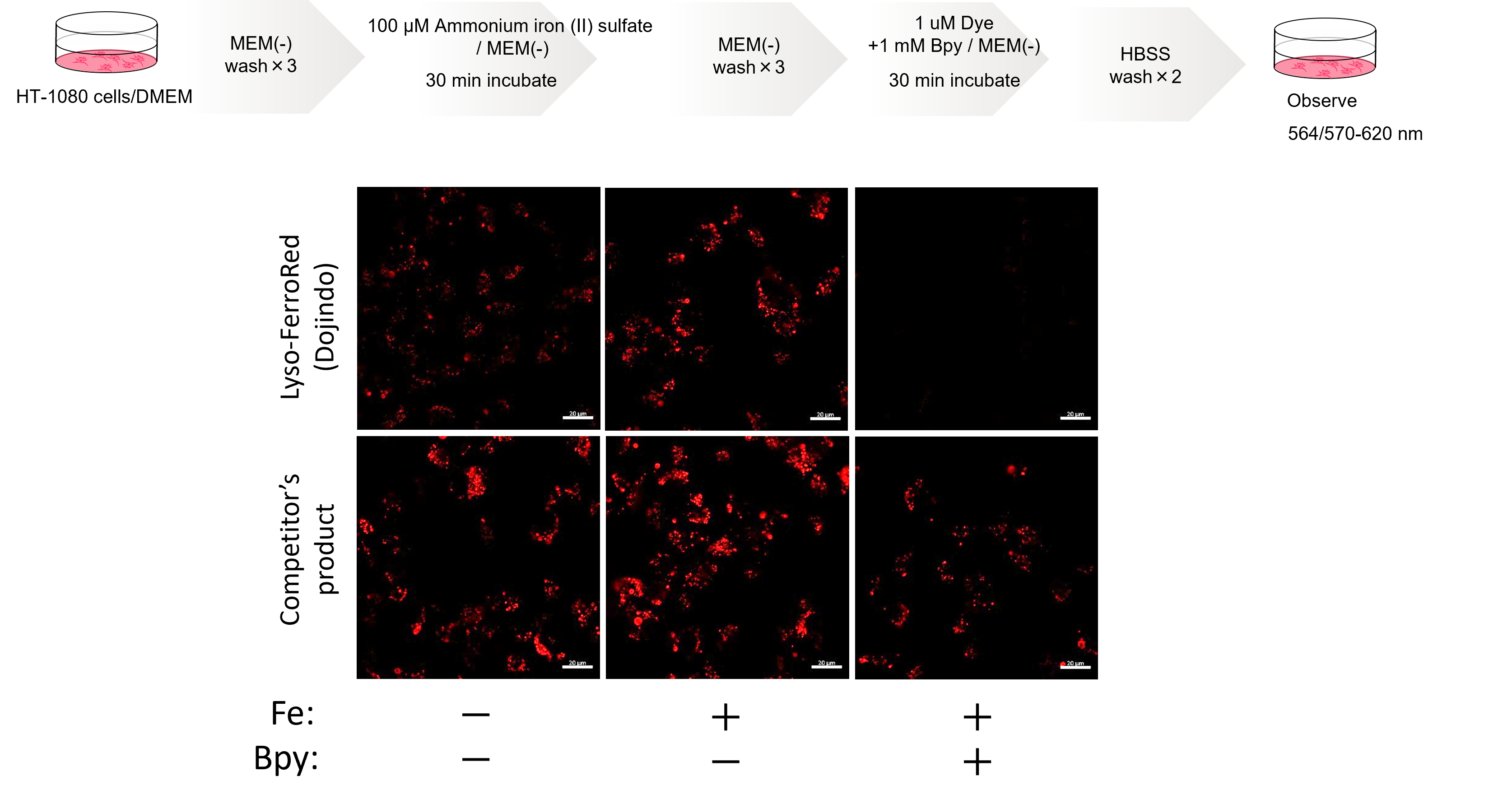
Comparative Performance Analysis
Our Lyso-FerroRed is highly purified and provides a clearer indication of intracellular ferrous ion changes than competitor lysosomal iron detection dyes.
In an experiment involving the addition of ferrous ions, ammonium iron (II) sulfate and the iron chelator Bpy, the fluorescence intensity of the Lyso-FerroRed responds to intracellular ferric ion changes and there is a lower background in the presence of Bpy.
In contrast, the competitor's product still shows signals when treated with Bpy, suggesting that it may show signals unrelated to intracellular ferrous ion.
Experimental example: Changes in Each Indicator Following the Co-treatment of Ferroptosis Inducers and Lysosomal Inhibitors
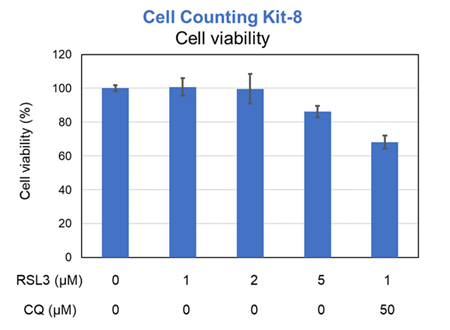
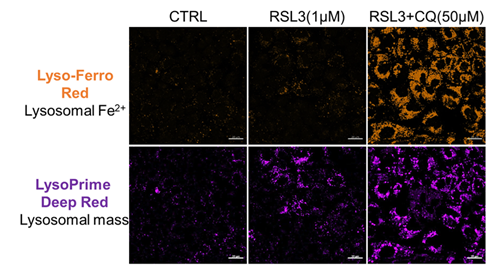
Previous studies have suggested that ferrotosis susceptibility varies among cancer cell lines. It has also been reported that increasing lysosomal stress in ferrotosis-resistant cancer cells can promote ferrotosis*. We treated A549 cells, which exhibit ferroptosis resistance, with the ferroptosis inducer RSL3 or RSL3 combined with the lysosomal inhibitor chloroquine (CQ) for 24 hours. Then, we analyzed changes in cell viability, lysosomal Fe²⁺, and lysosomal content. Treatment with RSL3 alone did not significantly alter cell viability or intracellular Fe²⁺ levels; however, some lysosomal aggregation (strong LysoPrime Deep Red signal) was observed. In contrast, cells treated with both RSL3 and CQ simultaneously exhibited increased intracellular Fe²⁺ levels, lysosomal enlargement, and decreased cell viability, which is consistent with previous reports. These results suggest that increased intracellular Fe²⁺ may promote ferroptosis.
* Saimoto. Y, et al., Nature Communications, 2025, 16, 3554.
[Products in use]
Cell viability: Cell Counting Kit-8 (Product code: CK04)
Lysosomal Fe2+: Lyso-FerroRed (Product code: L270)
Lysosomal mass: LysoPrime Deep Red - High Specificity and pH Resistance (Product code: L264)
References
| No. | Sample | Instruments | Publications |
|---|---|---|---|
| 1) | Cell (HepG2) |
Fluorescence microscope | Niwa, M., Hirayama, T., Okuda, K., Nagasawa, H., "A New Class of High-Contrast Fe(II) Selective Fluorescent Probes Based on Spirocyclized Scaffolds for Visualization of Intracellular Labile Iron Delivered by Transferrin", Org. Biomol. Chem. 2014, 12 (34), 6590–6597. DOI: 10.1039/C4OB00935E |
| 2) | Cell (Calu-1) |
Fluorescence microscope | Saimoto, Y., Kusakabe, D., Morimoto, K., Matsuoka, Y., Kozakura, E., Kato, N., Tsunematsu, K., Umeno. T., Kiyotani, T., Matsumoto, S., Tsuji, M., Hirayama, T., Nagasawa. H., Uchida, K., Karasawa, S., Jutanom, M., Yamada, K., "Lysosomal lipid peroxidation contributes to ferroptosis induction via lysosomal membrane permeabilization", Nat Commun, 2025, 16, 3554. DOI: 10.1038/s41467-025-58909-w |
Q & A
-
Q
How many samples can be measured by L270?
-
A
The number of measurable samples is as follows:
・ 96-well plate: 3 plates
・ ibidi 8-well plate: 21 plates
・ 35 mm dish: 17 dishes
・ 6-well plate: 17 wells
-
Q
Are there any experimental examples that serve as positive controls?
-
A
The manual includes examples of detection using HT-1080 cells incorporating ammonium iron sulfate. Please refer to steps 1-6 in the experimental examples section.
-
Q
Are there any precautions to take when preparing the working solution?
-
A
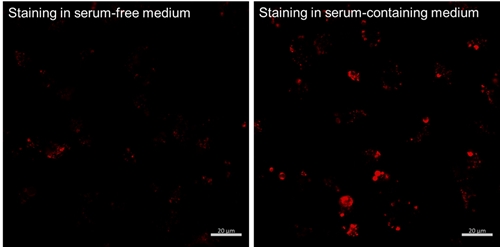
Prepare using HBSS or serum-free medium. Preparing in serum-containing medium causes the iron in the serum to react with the dye, resulting in increased background fluorescence.
-
Q
Are there any precautions to take when detecting with a flow cytometer?
-
A
Typically, trypsin is used to detach cells from dishes after staining with low-molecular-weight fluorescent probes. After detaching cells with trypsin, cells are recovered using serum-containing medium to quench residual trypsin.
Lyso-FerroRed reacts with iron in serum when it comes into contact with serum-containing medium. Therefore, when detaching cells, scrape them off using a scraper and recover them in PBS or HBSS.
-
Q
Is this dye compatible with fixed cells?
-
A
No.
Lyso-FerroRed signal is undetected and the changes in intracellular iron(II) ions cannot be confirmed in fixed cells.HT-1080 cells treated with ammonium iron(II) sulfate (final concentration: 100 μmol/L) were stained with Lyso-FerroRed, then observed under a fluorescence microscope after either no fixation or 30 minutes of fixation (4% PFA/PBS).
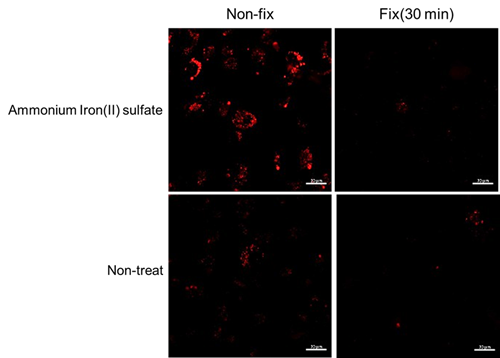
[Dtection condition]
Ex : 564 nm(0.8 %), Em : 570-620 nm
Gain : 480 V
Handling and storage condition
| Fluorescence spectrum: | To pass test |
|---|---|
| Confirmation test: | To pass test |
| Ambient temperature, Protect from light |