3-Deoxyglucosone
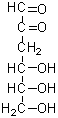
Stress Marker Detection
-
Product codeD535 3-Deoxyglucosone
-
CAS No.4084-27-9
-
Chemical name3-Deoxy-D-erythro-hexos-2-ulose
-
MWC6H10O5=162.14
| Unit size | Price | Item Code |
|---|---|---|
| 1 mg | $163.00 | D535-08 |
Product Description
Advanced glycation end-products (AGEs) have been studied as one of the causes of diabetic complications. Several compounds have been identified as AGEs, including pyralline, pentosidine, imidazolone, and pyropyridine. Glyoxal and methylglyoxal are reactive dicarbonyl compounds generated by glucose self-oxidation that are known to be AGE precursors. Another dicarbonyl compound, 3-Deoxyglucosone (3-DG), is also known to be one of the AGE precursors. 3-DG is derived from the Amadori rearrangement products of proteins and sugars in early stages of the Maillard reaction. 3-DG is also derived from fructose, which is present in high levels in diabetic patients, by a selfcondensation reaction. Fructose-3-phosphate has been found to enhance cross-linking reactions of lens proteins in a diabetic rat model. Therefore, 3-DG derived from fructose-3-phosphate has been studied as a possible cause of cataracts. Dr. Miyata and others reported that the 3-DG serum level in a diabetic rat model was 918 nM (normal level: 379 nM) and it was suppressed to 695 nM after 3 weeks of feeding aminoguanidine (50 mg/kg/day), an inhibitor of protein glycation. This suggests that compounds with 3-DG quenching activity may have clinical uses. 3-DG may be involved in other diseases as well. Dr. Niwa and others reported that uremia patients had elevated 3-DG levels, and that the 3-DG levels of diabetic uremia patients were even higher. There is also evidence that 3-DG inhibits DNA synthesis, suppressing cell proliferation as a consequence. Though several roles of 3-DG have become clear, many remain unknown. Glyoxal and methylglyoxal are other reactive dicarbonyl compounds generated by glucose self-oxidation that are known to be AGE precursors. There are two methods for determining 3-DG levels: HPLC and mass spectrometry (MS). However, there is some discrepancy between the HPLC and MS methods when measuring 3-DG levels in vivo. HPLC analysis is based on a fluorescent compound, 2-(2,3,4-trihydroxybutyl)-benzo[g]quinoxaline, generated by a coupling reaction between 3-DG and 2,3-diaminonaphthalene. Analogs of 2,3-diaminonaphthalene, such as 1,2-diamino-4,5-dimethoxy-benzene and 1,2-diamino-4,5-methylenedioxybenzene, can also be used. 3-DG can be utilized for AGE production or as a standard for 3-DG level detection in plasma or serum samples.
References
1) F. Hayase, R. H. Nagaraj, S. Miyata, F. G. Njoroges and V. M. Monnier, "Aging of Proteins: Immunological Detection of a Glucose-derived Pyrrole Formed during Maillard Reaction in Vivo", J. Biol. Chem., 1989, 264, 3758.
2) S. Miyata and V. Monnier, "Immunohistochemical Detection of Advanced Glycosylation End Products in Diabetic Tissue Using Monoclonal Antibody to Pyrraline", J. Clin. Invest., 1992, 89, 1102.
3) S. Taneda and V. M. Monnier, "ELISA of Pentosidine, an Advanced Glycation End Product, in Biological Specimens", Clin. Chem., 1994, 40, 1766.
4) D. G. Dyer, J. A. Blackedge, S. R. Thorpe and J. W. Baynes, "Formation of Pentosidine during Nonenzymatic Browning of Proteins by Glucose", J. Biol. Chem., 1991, 266, 11654.
5) T. Shinoda, F. Hayase and H. Kato, "Suppres
sion of Cell-cycle Progression during the S Phase of Rat Fibroblasts by 3-Deoxyglucosone, a Maillard Reaction Intermediate", Biotechnol. Biochem., 1994, 58, 1936.
6) F. Hayase, Y. Konishi and H. Kato, "Identification of the Modified Structure of Arginine Residue in Proteins with 3-Deoxyglucosone, a Maillard Reaction Intermediate", Biosci. Biotechnol. Biochem., 1995, 59, 1407.
7) T. Niwa, "3-Deoxyglucosone: Metabolism, Analysis, Biological Activity, and Clinical Implication.", J Chromatogr. B. Biomed Sci. Appl., 1999, 731, 23.
8) D. V. Zyzak, J. M. Richardson, S. R. Thoepe and J. W. Baynes, "Formation of Reactive Intermediates from Amadori Compounds under Physiological Conditions", Arch. Biochem. Biophys., 1995, 316, 547.
9) B. S. Szwergold, F. Kappler and T. R. Brown, "Identification of Fructose 3-Phosphate in the Lens of Diabetic Rats", Science, 1990, 247, 451.
Handling and storage condition
| Appearance: | White to pale yellow solid |
|---|---|
| Purity (HPLC): | ≧ 99.0 % |
| IR spectrum: | Authentic |
| -20°C, Nitrogen substitution, Protect from moisture |








