General Information
Nutrient metabolism is necessary for energy production in cells and regulates various cellular functions, including gene expression. Glucose is a key substrate for the generation of ATP and the maintenance of cellular homeostasis. Thus, glucose metabolism has been the subject of intense investigations. In cancer research, tumor cells increase glucose uptake and consumption to support their growth and proliferation. Therefore, elevated glucose uptake is a marker of tumors, and glucose transporters are important targets in cancer treatment.
The Glucose Uptake Probe-Green included in this kit is taken up into cells via glucose transporters. This kit enables the analysis of glucose uptake ability of cells using microplate measurement.The Quenching Buffer that eliminates the fluorescence of any extracellular Glucose Uptake Probe-Green is included in this kit, allowing for easy measurement of actual glucose uptake ability without the need for cell washing.
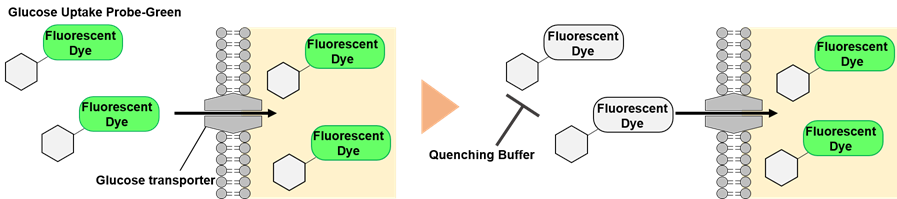
Figure 1. Principle of the glucose uptake measurement using Glucose Uptake Plate Assay Kit
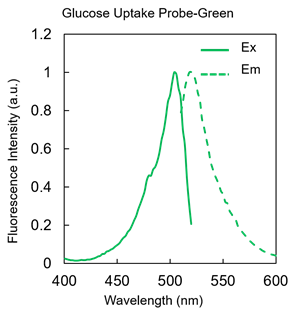
Figure 2. Excitation and emission spectra of Glucose Uptake Probe-Green
Kit Contents
| 100 tests | 500 tests | |
| Glucose Uptake Probe-Green | × 1 | × 5 |
| Quenching Buffer | 11 ml × 1 | 11 ml × 5 |
Storage Conditions
Store at 0–5°C
Required Equipment and Materials
- 20–200 µl multichannel pipette
- 100–1000 µl、20–200 µl、2–20 µl micropipettes
- Dimethyl sulfoxide (DMSO)
- Medium (glucose- and serum-free)
- 1.5 ml microtube
- fluorescence microplate reader
- 96-well black microplate (clear bottom)
Precautions
- Equilibrate reagents to room temperature before use.
- Briefly centrifuge the Glucose Uptake Probe-Green tube before opening to collect the content at the bottom.
- Analysis of samples in triplicate is recommended for accuracy.
- Pre-warm the medium (glucose- and serum-free), probe working solution, and Quenching Buffer to around 37°C in an incubator or water bath. Glucose uptake into cells may be affected by the temperature of the culture medium, probe working solution, and Quenching Buffer.
- When you use cells that are prone to detaching from the plate, please refer to experimental example 3.
Preparation of Solutions
1. Preparation of a probe stock solution
Add 40 μl of DMSO to the Glucose Uptake Probe-Green tube and dissolve by pipetting and vortex mixing.
- Store the probe stock solution at -20°C and protect it from light. The probe stock solution is stable for 1 month.
2. Preparation of a probe working solution
Dilute the probe stock solution 500-fold with glucose- and serum-free medium.
- The optimal concentration of the probe working solution varies depending on cell type. Please consider the optimal concentration (range: x250–x1000).
General Protocol
- Seed cells on a microplate. Culture the cells at 37°C overnight in a 5% CO2 incubator.
- Remove the culture medium and wash the cells with pre-warmed glucose- and serum-free medium twice.
- Pre-warm the culture medium in an incubator or water bath (37°C).
- When using cells that are prone to detaching from the plate, please use a Poly-D-Lysine (PDL)-coated plate (see Experimental Example 3).
- Add pre-warmed glucose- and serum-free medium to each well and incubate at 37°C for 15 min in a 5% CO2 incubator.
- Pre-warm the culture medium in an incubator or water bath (37°C).
- Remove the supernatant and add pre-warmed probe working solution to each well. Incubate at 37°C for 15 min in a 5% CO2 incubator.
- Pre-warm the probe working solution in an incubator or water bath (37°C).
- The optimum staining time varies depending on the cell types. Please consider the optimal time (range: 15–60 min).
- Add an equal amount of the pre-warmed Quenching Buffer to each well as the probe working solution.
- Pre-warm the Quenching Buffer in an incubator or water bath (37°C).
- Measure the fluorescence intensity using a microplate reader (bottom reading, Ex/Em = 488/520 nm).
- Please measure within 10 min after adding the Quenching Buffer, because the fluorescence intensity will decrease over time.
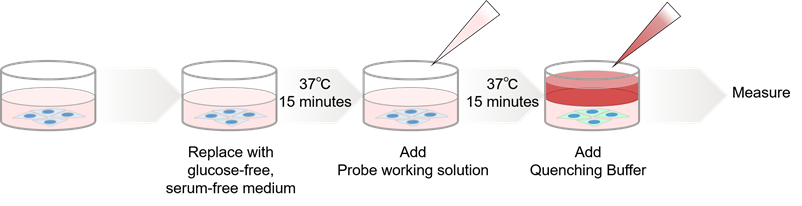
Figure 3. Procedure for the glucose uptake measurement using Glucose Uptake Plate Assay Kit
- For detailed experimental procedure, see the General Protocol.
Experimental Example 1
Competitive inhibition of the probe uptake with D-glucose (A549 cells)
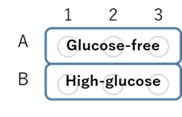
Figure 4. Plate format
- A549 cell suspensions (2.0 × 105 cells/ml, 200 µl/well) in DMEM (10% fetal bovine serum、1% penicillin-streptomycin) were seeded in a 96-well microplate (655090, Greiner GmbH) and cultured at 37°C overnight in a 5% CO2 incubator.
- After removing the supernatant, the cells were washed twice with 200 μl of pre-warmed DMEM (glucose- and serum-free, 37°C).
- Pre-warmed DMEM (200 μl, glucose- and serum-free, 37°C) was added to each well, and the cells were incubated at 37°C for 15 min in a 5% CO2 incubator.
- After removing the supernatant, 100 μl of pre-warmed probe working solution in DMEM (glucose- and serum-free, 37°C) or DMEM (high-glucose, serum-free, 37°C) was added to each well (see Figure 4) and the cells were incubated at 37°C for 30 min in a 5% CO2 incubator.
- The pre-warmed Quenching Buffer (100 µl, 37°C) was added to each well.
- The fluorescence intensity was measured using a microplate reader (Infinite M200 PRO, Tecan Trading AG, bottom reading, Ex/Em = 488/520 nm).
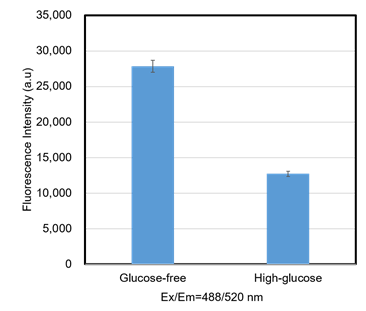
Figure 5. Competitive inhibition of the probe uptake with D-glucose (A549 cells)
In A549 cells, glucose competitively inhibited probe uptake, thereby decreasing the fluorescence intensity.
Experimental Example 2
Inhibition of the probe uptake with cytochalasin B (HepG2 cells)

Figure 6. Plate format
- HepG2 cells suspension (2.0 × 105 cells/ml, 200 µl/well) in DMEM (10% fetal bovine serum、1% penicillin-streptomycin) were seeded in a 96-well microplate (ib89626, ibidi GmbH) and cultured at 37°C overnight in a 5% CO2 incubator.
- DMEM (100 μl, 10% fetal bovine serum, 1% penicillin–streptomycin) containing 0 or 7.5 μmol/l cytochalasin B (final conc. 2.5 µmol/l cytochalasin B) was added to each well (see Figure 6), and the cells were incubated at 37°C overnight in a 5% CO2 incubator.
- After removing the supernatant, the cells were washed twice with 200 μl of pre-warmed DMEM (glucose- and serum-free, 37°C).
- Pre-warmed DMEM (200 μl, glucose- and serum-free, 37°C) was added to each well, and the cells were incubated at 37°C for 15 min in a 5% CO2 incubator.
- After removing the supernatant, 100 μl of pre-warmed probe working solution in DMEM (glucose- and serum-free, 37°C) was added to each well, and the cells were incubated at 37°C for 15 min in a 5% CO2 incubator.
- The pre-warmed Quenching Buffer (100 µl, 37°C) was added to each well.
- The fluorescence intensity was measured using a microplate reader (Infinite M200 PRO, bottom reading, Ex/Em = 488/520 nm).
- The fluorescent intensity of the Glucose Uptake Probe-Green was normalized by the fluorescent intensity of Hoechst 33342.
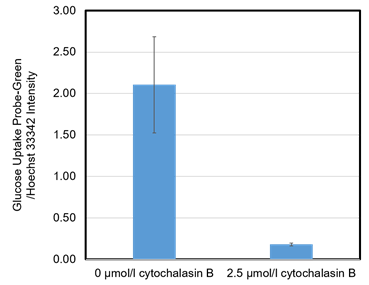
Figure 7. Inhibition of the probe uptake with cytochalasin B (HepG2 cells)
The probe uptake was inhibited by cytochalasin B (an inhibitor of glucose transporter) treatment.
Experimental Example 3
Inhibition of the probe uptake with 2-Deoxy-D-glucose (HEK293 cells)

Figure 8. Plate format
- Poly-D-Lysine (PDL) solution (1 mg/ml) was diluted 10-fold with PBS(-).
- The diluted PDL solution (150 µl/well) was added to each well of a 96-well microplate (655090, Greiner GmbH), and the plate was incubated at room temperature for 1 hour.
- Each well was washed three times with PBS(-) (200 µl/well).
- HEK293 cells suspension (6.0 × 105 cells/ml, 200 µl/well) in MEM (10% fetal bovine serum、1% penicillin-streptomycin) were seeded in the PDL-coated 96-well microplate and cultured at 37°C overnight in a 5% CO2 incubator.
- 2-Deoxy-D-glucose (2-DG) solution (900 mmol/l, 22 µl) was added to each well (see Figure 8) (final conc.100 mmol/l 2-DG), and the cells were incubated at 37°C for 2.5 hours in a 5% CO2 incubator.
- After removing the supernatant, the cells were washed twice with 200 μl of pre-warmed DMEM (glucose- and serum-free, 37°C).
- Pre-warmed DMEM (200 μl, glucose- and serum-free, 37°C) was added to each well, and the cells were incubated at 37°C for 15 min in a 5% CO2 incubator.
- After removing the supernatant, 100 μl of pre-warmed probe working solution in DMEM (glucose- and serum-free, 37°C) was added to each well, and the cells were incubated at 37°C for 30 min in a 5% CO2 incubator.
- The pre-warmed Quenching Buffer (100 µl, 37°C) was added to each well.
- The fluorescence intensity was measured using a microplate reader (Infinite M200 PRO, Tecan Trading AG, bottom reading, Ex/Em = 488/520 nm).
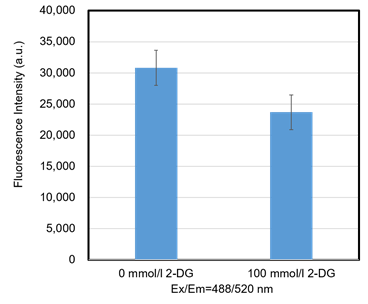
Figure 9. Inhibition of the probe uptake with 2-DG (HEK293 cells)
The probe uptake was inhibited by 2-DG (an inhibitor of glucose metabolism) treatment.
UP08: Glucose Uptake Plate Assay Kit
Revised Aug., 27, 2025


 Hidden sections will not be printed.
Hidden sections will not be printed.