General Information
Transporters responsible for nutrient uptake have been attracting attention as a target for cancer diagnosis and drug discovery. For example, L-type amino acid transporter 1 (LAT1), which is one of the amino acid (AA) transporters, is specifically expressed in many cancer cells. To assess an AA transporter activity, an assay using radioisotope-labeled AAs has been used as a conventional method. However, this method requires special handling and disposal of radioactive materials.
Amino Acid Uptake Assay Kit allows a simple fluorescence assay for AA transporter activity using Boronophenylalanine (BPA), which is an AA analog used in boron neutron capture therapy for cancer, and the fluorescent probe in this kit. This method can be applied to fl uorescence imaging, microplate measurement, and fl ow cytometry, and is thus useful for evaluating cellular AA uptake activity and screening AA transporter inhibitors.
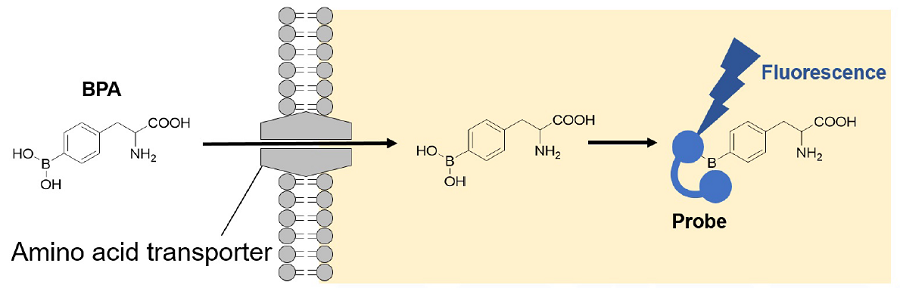
Figure 1. Principle of Amino Acid Uptake Assay Kit
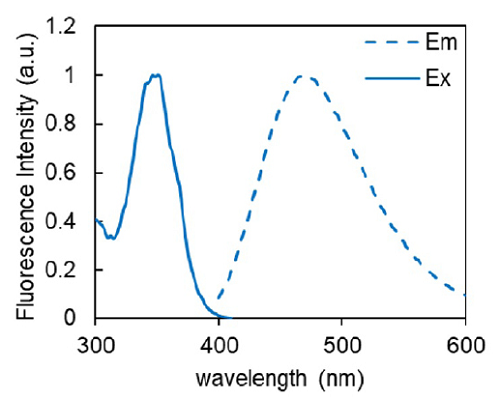
Figure 2. Excitation and emission spectra of Probe-BPA complex
Kit Contents
| 20 tests | 100 tests | |
| BPA Solution | 35 μl x 1 | 175 μl x 1 |
| BPA Dilution Buffer | 35 μl x 1 | 175 μl x 1 |
| Probe Solution | 15 μl x 1 | 75 μl x 1 |
Storage Condition
Store at -20°C, and protect from light.
Required Equipment and Materials
- 96 well black microplate
- Please refer to Q&A for available plates.
- 20-200 μl multichannel pipette
- Micropipette
- 15 ml conical tube
- 1.5 ml microtube
- Hanks’ Balanced Salt Solution (HBSS) or PBS(+) with 0.1% glucose
Precaution
- Please tap the tube before opening and open it with care. The content may have relocated from the bottom of the tube during the shipping.
Preparation of Solutions
Preparation of BPA uptake solution
Mix BPA Solution with an equal volume of BPA Dilution Buffer. Then, dilute this solution 50-fold with HBSS.
| Adherent Cells | Suspension Cells | ||||
| Culture equipment (amount) |
6-well (1.5 ml/well) |
24-well (0.3 ml/well) |
96-well (0.15 ml/well) |
35-mm Dish (1.5 ml/well) |
1.5 ml microtube (0.5 ml/well) |
| HBSS | 1500 μl | 300 μl | 150 μl | 1500 μl | 500 μl |
| BPA Solution | 15 μl | 3 μl | 1.5 μl | 15 μl | 5 μl |
| BPA Dilution Buffer |
15 μl | 3 μl | 1.5 μl | 15 μl | 5 μl |
- PBS(+) with 0.1% glucose can be used instead of HBSS.
- Prepare the BPA uptake solution fresh each day and use it immediately after preparation.
Preparation of working solution
Dilute Probe solution 250-fold with HBSS and mix by pipetting and vortex mixing.
| Adherent Cells | Suspension Cells | ||||
| Suspension Cells Culture equipment (amount) |
6-well (1.5 ml/well) |
24-well (0.3 ml/well) |
96-well (0.15 ml/well) |
35-mm Dish (1.5 ml/well) |
1.5 ml microtube (0.5 ml/well) |
| HBSS | 1500 μl | 300 μl | 150 μl | 1500 μl | 500 μl |
| Probe Solution | 6 μl | 1.2 μl | 0.6 μl | 6 μl | 2 μl |
Centrifuge the working solution at 300 x g for 3 min, transfer the supernatant to a new tube.
- The undissolved residue of the probe affects observation under a fluorescence microscope and fluorescence measurement with a microplate reader.
- Prepare the working solution fresh each day and use it immediately after preparation.
General Protocol
Fluorescence Imaging, Plate reading
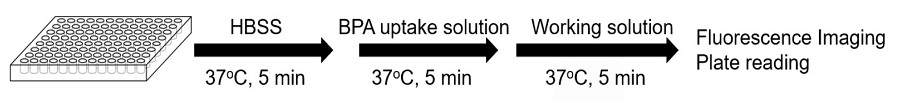
Figure 3. Protocol for fluorescence imaging and plate reading
- Use a multichannel pipette to reduce the time difference between each well
- For detailed experimental procedures, see the General Protocol.
- Seed cells in a 96-well microplate and culture the cells at 37°C overnight in a 5% CO2 incubator.
- Remove the culture medium and wash the cells with pre-warmed HBSS (37℃*1) three times.
- Add 150 μl of pre-warmed HBSS (37℃*1) and incubate at 37°C for 5 minutes in a 5% CO2 incubator.
- Remove the supernatant, add 150 μl of pre-warmed BPA uptake solution (37℃*1) or pre-warmed HBSS (37℃*1, blank) and incubate at 37°C for 5 minutes in a 5% CO2 incubator.
- Remove the supernatant and wash the cells with pre-warmed HBSS (37℃*1) three times.
- Remove the supernatant, add 150 μl of pre-warmed working solution (37℃*1) and incubate at 37°C for 5 minutes.
- *2 Observe under a fluorescence microscope or measure the fluorescence intensity with a fluorescence plate reader.
(Fluorescence Imaging: DAPI filter set, Plate reading: Ex/Em = 360/460 nm)
- Amino Acid uptake into the cells may be aff ected by the temperature. Pre-warm HBSS, BPA uptake solution and working solution in an incubator (37°C).
- Do not remove the working solution. The fluorescent signal disappears if the working solution is removed.
Keep the working solution in the wells during the observation and measurements.
Flow cytometry
For adherent cells
- Seed cells in a 6-well microplate at 2 x 105 cells/well and culture the cells at 37°C overnight in a 5% CO2 incubator.
- Remove the supernatant and wash the cells with pre-warmed HBSS (37℃*1) three times.
- Add 1,500 μl of pre-warmed HBSS (37℃*1) and incubate at 37°C for 5 minutes in a 5% CO2 incubator.
- Remove the supernatant, add 1,500 μl of pre-warmed BPA uptake solution (37℃*1) or pre-warmed HBSS (37℃, blank) and incubate at 37°C for 5 minutes in a 5% CO2 incubator.
- Remove the supernatant and wash the cells with pre-warmed HBSS (37℃*1) three times.
- Remove the supernatant, add 1,500 μl of pre-warmed working solution (37℃*1) and incubate at 37°C for 5 minutes.
- Harvest the cells using a cell scraper.
- Analyze the cells using a flow cytometer.
- Amino Acid uptake into the cells may be aff ected by the temperature. Pre-warm HBSS, BPA uptake solution and working solution in an incubator (37°C).
For suspension cells
- Dispense the cells to microtubes at 2 x 105 cells/tube.
- Centrifuge the tubes at 300 x g for 5 minutes, remove the supernatant and add 500 μl of pre-warmed HBSS (37℃*1).
Repeat this step twice. - Centrifuge the tubes at 300 x g for 5 minutes and remove the supernatant. Add 500 μl of pre-warmed HBSS (37℃*1), suspend by pipetting and incubate for 5 minutes at 37°C.
- Centrifuge the tubes at 300 x g for 5 minutes and remove the supernatant.
- Add 500 μl of pre-warmed BPA uptake solution (37℃*1) or pre-warmed HBSS (37℃*1, blank), suspend by pipetting and incubate for 5 minutes at 37°C.
- Centrifuge the tubes at 300 x g for 5 minutes, remove the supernatant and add 500 μl of pre-warmed HBSS (37℃*1).
Repeat this step twice. - Centrifuge the tubes at 300 x g for 5 minutes and remove the supernatant. Add 500 μl of pre-warmed working solution (37℃*1), suspend by pippeting and incubate for 5 minutes at 37°C.
- Analyze the cells using a flow cytometer.
- Amino Acid uptake into the cells may be aff ected by the temperature. Pre-warm HBSS, BPA uptake solution and working solution in an incubator (37°C).
Experimental example
Inhibition of amino acid uptake by BCH (2-Aminobicyclo [2.2.1] heptane-2-carboxylic acid) (HeLa cells)
Fluorescence imaging and plate reading
- HeLa cells (1 x 104 cells/well) in MEM (10% FBS) were seeded in a 96-well black microplate (ib89626, ibidi) and cultured at 37°C overnight in a 5% CO2 incubator.
- After removing the supernatant, the cells were washed three times with 150 μl of HBSS (37°C).
- HBSS containing 0 mmol/l (37°C, sample 1) or 1 mmol/l BCH (37°C, sample 2) were added and the cells were incubated at 37°C for 5 minutes in a 5% CO2 incubator.
- After removing the supernatant, BPA uptake solution containing 0 mmol/l BCH (37°C, sample1) , 1 mmol/l BCH (37°C, sample2) or HBSS (37°C, blank) were added and the cells were incubated at 37°C for 5 minutes in a 5% CO2 incubator.
- After removing the supernatant, the cells were washed with HBSS (37°C) three times.
- After removing the supernatant, working solution (37°C) was added and the cells were incubated at 37°C for 5 minutes in a 5% CO2 incubator.
- The cells were observed under a fluorescence microscope and the fluorescence intensity was measured with a microplate reader.
(Fluorescence Imaging: DAPI filter set, Plate reading: Ex/Em = 360/460 nm)
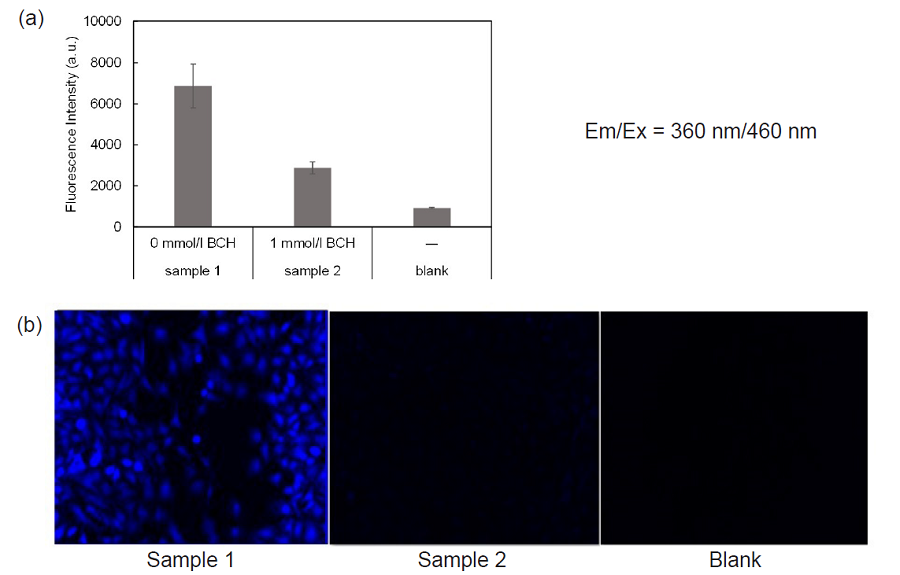
Figure 4. Inhibition of amino acid transporters activity by BCH
(a) microplate reading, (b) fl uorescence Imaging
For fl ow cytometry
- HeLa cells (1.5 x 105 cells/well) in MEM (10% FBS) were seeded in a 6-well microplate (3810-006, AGC techno glass) and cultured at 37°C overnight in a 5% CO2 incubator.
- After removing the supernatant, the cells were washed three times with 1,500 μl of HBSS (37°C).
- HBSS containing 0 mmol/l (sample 1) or 1 mmol/l BCH (sample 2) were added and the cells were incubated at 37°C for 5 minutes in a 5% CO2 incubator.
- After removing the supernatant, BPA uptake solution (37°C) including 0 mmol/l BCH (sample1), 1 mmol/l BCH (sample2) or HBSS (37°C, blank) were added and the cells were incubated at 37°C for 5 minutes in a 5% CO2 incubator.
- After removing the supernatant, the cells were washed with HBSS (37°C) three times.
- After removing the supernatant, working solution (37°C) was added and the cells were incubated at 37°C for 5 minutes in a 5% CO2 incubator.
- The cells were harvested using a cell scraper and analyzed with a flow cytometer (LSR Fortessa X-20, BD).
BV421 filter set (Ex:405 nm, Em:450/40 nm)
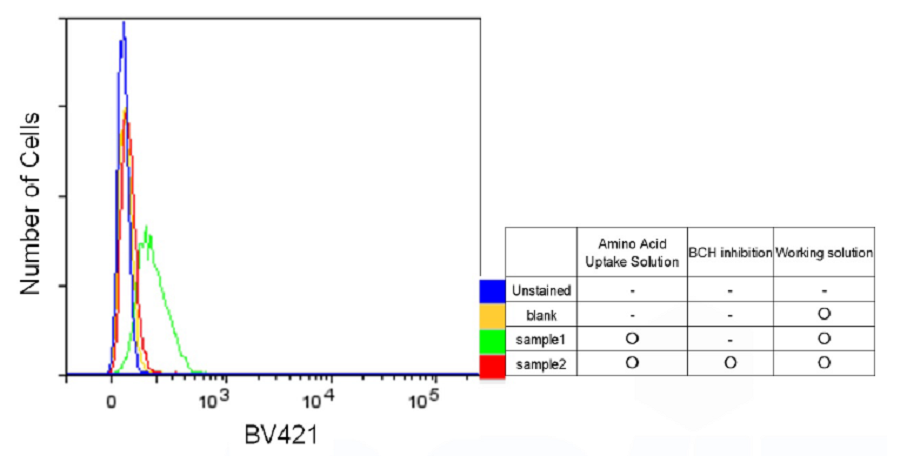
Figure 5. Inhibition of amino acid transporters activity by BCH (Flow cytometry)
This product was developed by the kind support of Ph.D. Mitsunori Kirihata at Research Center of Boron Neutron Capture Therapy, Research Organization for the 21st Century, Osaka Prefecture University.
Frequently Asked Questions / Reference
UP04: Amino Acid Uptake Assay Kit
Revised Mar., 05, 2025


 Hidden sections will not be printed.
Hidden sections will not be printed.

