General Information
DNA damage in normal cells is caused by repeated cell division and oxidative stress. Cellular senescence, a state of irreversible growth arrest, can be triggered to prevent DNA-damage.
Senescence-associated β-galactosidase(SA-β-gal), which is overexpressed in senescent cells, has been widely used as a marker of cellular senescence 1, 2).
The kit enables simple determination of cellular senescence by measuring SA-β-gal activity using a fluorometric substrate, SPiDER-βGal 3).
The assay system can be combined with widely used normalization methods (e.g. using a hemocytometer, BCA assay, and nucleic acid stains).
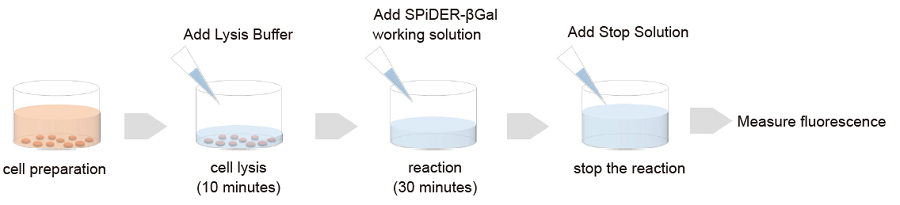
Figure 1 Assay procedure
Kit Contents
| 20 tests | 100 tests | |
| SPiDER-βGal | 1 tube | 5 tubes |
| Lysis Buffer | 40 mL x 1 | 100 mL x 2 |
| Assay Buffer | 1.5 mL x 1 | 7.5 mL x 1 |
| Stop Solution | 3 mL x 1 | 15 mL x 1 |
Storage Condition
Store at 0–5 ℃ and protect from light.
Required Equipment and Materials
- Fluorometer
- 96-well black plate
- Incubator (37 ℃ )
- Multi-channel pipette (20–200 μL)
- Micropipette (100–1000 μL, 20–200 μL)
- Phosphate buffered saline (PBS)
- Dimethylsulfoxide (DMSO)
- Conical tubes
Precautions
- Equilibrate reagents to room temperature prior to use.
- Centrifuge the tube (SPiDER-βGal) briefly before opening to remove all contents from the tube walls and inside the cap.
- Analyzing samples in triplicate is recommended for accuracy.
- Because the coloration reaction starts immediately after addition of the working solution to a well, use a multi-channel pipette to minimize experimental error by reducing the pipetting steps.
Preparation of Solutions
Preparation of SPiDER-βGal DMSO stock solution
Add 125 μL DMSO to the SPiDER-βGal tube and dissolve the contents using a vortex mixer.
- SPiDER-βGal is difficult to see by the naked eye because of the small amount.
- Vortexing is necessary to completely dissolve the contents (SPiDER-βGal).
- Store the SPiDER-βGal DMSO stock solution at -20℃ and protect from light (insert the bag). The prepared stock solution is stable at -20℃ for 1 month.
Preparation of SPiDER–βGal working solution
Prepare a 10-fold dilution of the stock solution in Assay Buffer.
- Prepare the working solution fresh each day.
- When using a 96-well plate, 50 μL of working solution is needed for each well.
- The working solution is light-sensitive. Protect it from light by covering the tube with aluminum foil.
General Protocol
SA-β-gal assay
- Seed cells on a plate or dish and culture at 37°C overnight in a 5% CO2 incubator.
- Perform suitable normalization for your experiment.
- If you need any assistance for normalization, please contact Dojindo’s technical support.
- Remove the supernatant and wash the cells with PBS once.
- Add Lysis Buffer and incubate the plate or dish at room temperature for 10 minutes.
- For the amount of Lysis Buffer, please refef to Table 1
Table 1 Lysis Buffer amount to be added 96-well plate 24-well plate 6-well plate 10-cm dish Lysis Buffer 50 μL 400 μL 1 mL 1.5 mL - Transfer 50 μL lysate solution to each well of a 96-well black plate.
- Add 50 μL SPiDER–βGal working solution to each well and incubate at 37℃ for 30 minutes.
- If there isn't any signal difference between control and senescent cells, please extend the incubation time (30 minutes to overnight).
- Add 100 μL Stop Solution to each well.
- Measure fluorescence using a fluorometer (Ex: 500–540 nm; Em: 540–580 nm).
Experimental Examples
Determination of SA-β-gal in WI-38 cells
- Passage 3 and 19 WI-38 cells (1×104 cells/well, MEM containg 10% fetal bovine serum and 1% penicillin-streptomycin) were seeded on a 96-well black plate (clear bottom) and cultured at 37℃ overnight in a 5% CO2 incubator.
- Cell Count Normalization Kit (code: C544) was used for normalization, which is a nucleoic acid stain based normalization kit.
- For this kit, more infomation is available at our web-site.
- The supernatant was removed and the cells were washed with 100 μL PBS once.
- After addition of 50 μL Lysis Buffer to each well, the plate was incubated at room temperature for 10 minutes.
- SPiDER-βGal working solution (50 μL) was added to each well and the plate was incubated at 37℃ for 30 minutes.
- Stop Solution (100 μL) was added to each well.
- Fluorescence signals were measured using a fluorometer (Ex: 535 nm; Em: 580 nm).
- Normalized SA-β-Gal activity was determined using the Cell Count Normalization Kit.
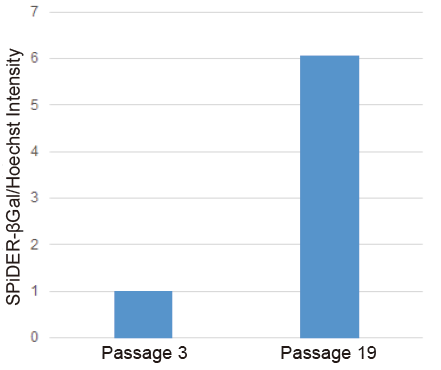
Figure 2 SA-β-gal activity in senescent WI-38 cells (microplate assay)
Determination of SA-β-gal in doxorubicin-treated WI-38 cells
- Passage 3 WI-38 cells (1×106 cells/dish, MEM containing 10% fetal bovine serum and 1% penicillin-streptomycin) were seeded on a 10-cm dish and cultured at 37℃ overnight in a 5% CO2 incubator.
- The medium was removed and the cells were washed with 10 mL PBS once.
- Doxorubicin solution (0.2 μmol/L in serum-free MEM) was added to the cells and the cells were cultured at 37℃ for 3 days in a 5% CO2 incubator.
- The supernatant was removed and the cells were washed with 10 mL PBS once.
- MEM (containg 10% fetal bovine serum and 1% penicillin-streptomycin) was added and the cells were cultured at 37℃ for 3 days in a 5% CO2 incubator.
- The medium was removed and the cells were washed with 10 mL PBS once.
- The doxorubicin-treated WI-38 cells (1×104 cells/well, MEM containg 10% fetal bovine serum and 1% penicillin-streptomycin) were seeded on a 96-well black plate (clear bottom) and cultured at 37℃ overnight in a 5% CO2 incubator.
- The doxorubicin-untreated WI-38 cells (1×104 cells/well, MEM containg 10% fetal bovine serum and 1% penicillin-streptomycin) were seeded on a 96-well black plate (clear bottom) and cultured at 37℃ overnight in a 5% CO2 incubator as control.
- Cell Count Normalization Kit (code: C544) was used for normalization, which is a nucleoic acid stain based normalization kit.
- The supernatant was removed and the cells were washed with 100 μL PBS once.
- After addition of 50 μL Lysis Buffer to each well, the plate was incubated at room temperature for 10 minutes.
- SPiDER-βGal working solution (50 μL) was added to each well and the plate was incubated at 37℃ for 30 minutes.
- Stop Solution (100 μL) was added to each well.
- Fluorescence signals were measured using a fluorometer (Ex: 535 nm; Em: 580 nm).
- Normalized SA-β-Gal activity was determined using the Cell Count Normalization Kit.
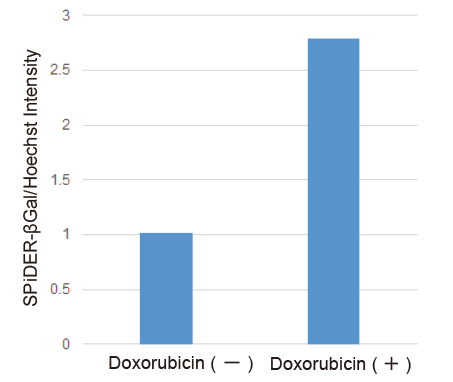
Figure 3 SA-β-gal activity in senescent WI-38 cells (microplate assay)
References
- Dimri, G. P. et al., Cell Biology, 1995, 92, 9363–9367.
- Park, A. M. et al., J. Biol. Chem., 2018, 293, DOI: 10.1074/jbc.RA118.003310
- Doura, T. et al., Angew. Chem., 2016, 55, 9620–9624.
Frequently Asked Questions / Reference
SG05: Cellular Senescence Plate Assay Kit - SPiDER-βGal
Revised Apr., 03, 2024


 Hidden sections will not be printed.
Hidden sections will not be printed.

