General Information
The gene of β-galactosidase from E. coli is widely used as a reporter gene assay marker. Although X-gal is well known reagent to detect β-galactosidase in cell or tissue samples, the assay using these reagents require to fix cells or tissues due to the poor cell-permeability. In addition, so far developed the assay using fluorescence reagents can not clearly differentiate β-galactosidase-expressed cells or regions.
To overcome these issues, Urano, Kamiya and co-workers have successfully developed SPiDER-βGal. SPiDER-βGal ideally possesses cell-permeability and the ability to retain in intracellular region.1)
By the enzymatic reaction, SPiDER-βGal immediately forms a quinone methide that acts as electrophile when proteins containing nucleophilic functional groups nearby the molecules. By the probe undergoes the reaction with a protein, the conjugates become fluorescent compounds. Thus, SPiDER-βGal allows a single-cell analysis because it does self-immobilizing to the intracellular proteins.
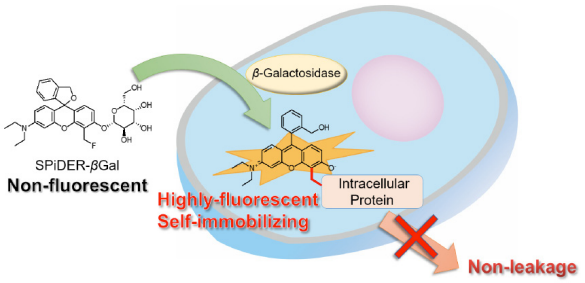
Fig. 1 Cell staining mechanism by SPiDER-βGal.
- < Recommended filter >
Ex:500 ~ 540 nm
Em:530 ~ 570 nm
There are internal detection examples obtained using confocal microscopy and flow cytometry at an excitation wavelength of 488 nm.
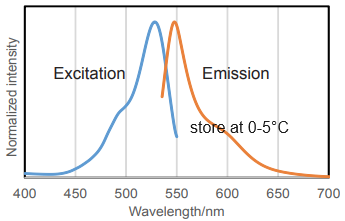
Fig. 2 Excitation and emission spectra of SPiDER-βGal after reaction with β-galactosidase
Contents
| SPiDER-βGal | 20 μg x 3 |
Storage Condition
Store at 0-5°C
- After a SPiDER-βGal is taken out from the seal bag, keep the unused SPiDER-βGal in the bag, seal tightly and store at 0-5°C.
Required Equipment and Materials
- Dimethyl sulfoxide (DMSO)
- Hanks’ HEPES buffer
- Micropipettes
- Microtubes
Preparation of solutions
Preparation of 1 mmol/l SPiDER-βGal DMSO stock solution
Add 35 μl of DMSO to a tube of SPiDER-βGal (20 μg) and dissolve it with pipetting.
- Note: Store the SPiDER-βGal stock solution at -20℃.
Preparation of 1 μmol/l SPiDER-βGal working solution
Dilute the SPiDER-βGal DMSO stock solution with Hanks’ HEPES buffer to prepare 1 μmol/l SPiDER-βGal working solution.
- Note: Hanks’ HEPES buffer is recommended to maintain cell condition.
General protocol
SPiDER-βGal staining
- Prepare cells for the assay.
- Discard the culture medium and wash the cells with Hanks’ HEPES buffer twice.
- Add an appropriate volume of SPiDER-βGal working solution.
- Incubate at 37℃ for 15 minutes.
- Observe the cells under a fluorescence microscope or by a flow cytometer.
- Note: After staining, the cells can be observed even without washing. However, you can wash it as needed.
Usage examples
Fluorescence microscopic detection of β-galactosidase-expressed cells
- HEK cells at 5 × 105 cells/ml (500 μl) and HEK/LacZ cells at 5 × 105 cells/ml (500 μl) were seeded in a 35 mm dish in DMEM (10% fetal bovine serum, 1% penicillin-streptmycin) and cultured overnight in a 5% CO2 incubator at 37℃.
- The cells were washed with 2 ml of Hanks’ HEPES buffer twice.
- SPiDER-βGal working solution (2 ml) was added to the culture dish, and the cells were incubated for 15 minutes at 37℃.
- After the supernatant was removed, the cells were washed Hanks’ HEPES buffer (2 ml) twice.
- Hanks’ HEPES buffer (2 ml) were added, and the cells were observed under a fluorescence microscope. (Fig. 3A)
- After the supernatant was removed, 4% paraformaldehyde (PFA) /PBS solution (2 ml) was added to the culture dish, and the cells were incubated for 15 minutes at room temperature.
- After 4% PFA/PBS solution was removed, the cells were washed Hanks’ HEPES buffer (2 ml) twice.
- Hanks’ HEPES buffer (2 ml) were added, and the cells were observed under a fluorescence microscope. (Fig. 3B)
Filter (wavelength/band pass)
Fluorescence imaging: 550/25 nm (Ex), 605/70 nm (Em)
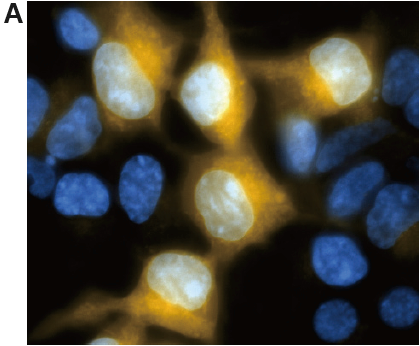
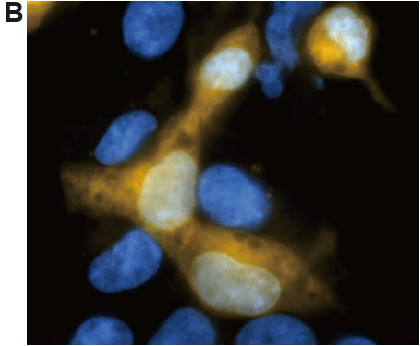
Fig. 3 Fluorescence imaging of HEK/LacZ cells and HEK cells at 1: 1 ratio.
A. living cell, B. fixing cells (4% PFA/PBS)
(yellow: SPiDER-βGal, blue: Hoechst 33342)
β-galactosidase-expressed cells (HEK/LacZ cells) were clearly observed in fluorescence imaging. In addition, the result was not changed by fixing the cells.
Flow cytometric detection of β-galactosidase-expressed cells
- HEK cells at 5 × 105 cells/ml (500 μl) and HEK/LacZ cells at 5 × 105 cells/ml (500 μl) were mixed in a microtube.
- SPiDER-βGal DMSO stock solution (1 μl) was added to the tube, and the cells were incubated for 15 minutes at 37°C.
- The cells were analyzed by a flow cytometer. (488 nm excitation, 530/30 nm bandpass filter)
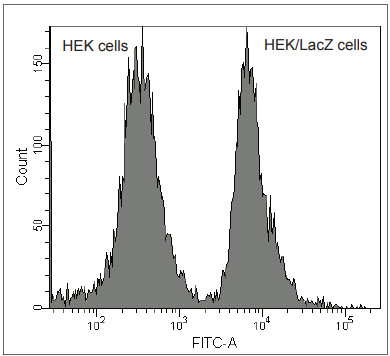
Fig. 4 Analysis of HEK/LacZ cells and HEK cells at 1: 1 ratio by flow cytometry.
β-galactosidase-expressed cells (HEK/LacZ cells) were clearly differentiate from HEK cells in flow cytometry data analysis.
Reference
- T. Doura, M. Kamiya, F. Obata, Y. Yamaguchi, T. Y. Hiyama, T. Matsuda, A. Fukamizu, M. Noda, M. Miura and Y. Urano, Angew. Chem. Int. Ed., 2016, 55, 9620.
Frequently Asked Questions / Reference
SG02: SPiDER-βGal
Revised Aug., 23, 2023


 Hidden sections will not be printed.
Hidden sections will not be printed.