General Information
It has been recognized that hydrogen sulfide (H2S) has an important role as a physiological active substance for vasodilation, cytoprotection, and modulation of insulin secretion. H2S is considered as a gaseous molecule such as nitric oxide and carbon monoxide. However, around 80% of the total sulfide exists as hydrogen sulfide anion (HS-) under physiological condition, since the pKa is about 7. In addition, HS- easily converts to various biochemical molecules such as persulfides and polysulfides, which react with sulfhydryl moieties in a living body. -SulfoBiotics- HSip-1 is a novel fluorescent probe to detect H2S selectively and it emits strong green fluorescence when it reacts with H2S.
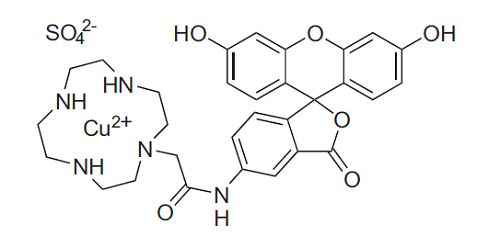
HSip-1
(MW: 719.22)
Fig. 1 Chemical structures of HSip-1
-
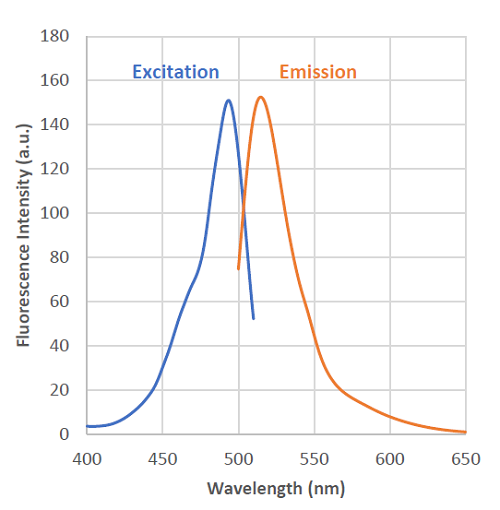
Fig. 2 Excitation and emission spectra of HSip-1 reacted with H2S
- λex : 491 nm
λem : 516 nm
<Recommended filter>
Ex:470 ~ 500 nm
Em:500 ~ 550 nm
Contents
| 1 mg | x 1 |
Storage Conditions
Store in a cool dark place.
Required Equipment and Materials
- Purified water
- PBS
- Micropipettes
Preparation of Solutions
Preparation of 10 mmol/l HSip-1 stock solution
Add 139 μl of purified water to a tube containing 1 mg of HSip-1 and dissolve it by pipetting.
- Store at -20 °C. The reconstituted solution is stable at -20°C for 1 month.
Experimental Example
Detection of hydrogen sulfide by HSip-1
- HSip-1 stock solution (10 mmol/l) was diluted with PBS to prepare 200 μmol/l HSip-1 working solution.
- Sodium Sulfide (-SulfoBiotics- Sodium Sulfide (Na2S), 7.8 mg) were dissolved in 1 ml of de-oxygenated H2O prepared by bubbling of nitrogen gas (100 mmol/l Na2S solution).
- Na2S solution (100 mmol/l, 20 μl) was added to 980 μL of de-oxygenated H2O to prepare 2 mmol/l Na2S solution.
- Na2S solution (2 mmol/l,100 μl) was added to 900 μl of de-oxygenated H2O to prepare 200 μmol/l Na2S solution.
- Na2S solution (200 μmol/l) was diluted with de-oxygenated H2O to prepare various concentrations of Na2S solution by serial dilution (200, 100, 50, 25, 12.5, 6.3, 3.2, 0 μmol/l).
- HSip-1 working solution (200 μmol/l, 350 μl) was added to 300 μl of the Na2S solutions and mixed using a vortex mixer.
- The solutions were incubated at room temperature for 30 minutes and 200 μl of the solution were transfered to each well (96-well plate).
- The fluorescence intensities were measured at 516 nm (λex=491 nm) with a microplate reader.
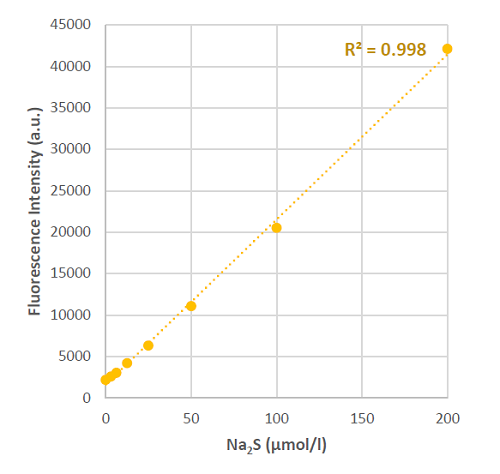
Fig. 3 Fluorescence intensity changes at 516 nm with various concentrations of hydrogen sulfide.
- Experimental example on HeLa cells is available. It can be found by searching “SB21“ on our website.
These products were commercialized under the advisory of Dr. Tetsuo Nagano and Dr. Kenjiro Hanaoka (The University of Tokyo).
Reference
- K. Sasakura, K. Hanaoka, N. Shibuya, Y. Mikami, Y. Kimura, T. Komatsu, T. Ueno, T. Terai, H. Kimura, and T. Nagano, “Development of a Highly Selective Fluorescence Probe for Hydrogen Sulfide”, J. Am. Chem. Soc., 2011, 133, 18003.
Frequently Asked Questions / Reference
SB21: -SulfoBiotics- HSip-1
Revised Nov., 16, 2023


 Hidden sections will not be printed.
Hidden sections will not be printed.