General Information
Modification of protein thiols is one of the most important post-translational modifications and it occurs according to the redox states in cells. It has recently been revealed that the modifications of thiol groups control cellular functions such as transcription, protein expression, cell death, etc. Therefore, detection of the redox states of the individual protein is important to understand cellular events.
-SulfoBiotics- PEG-PCMal is a reagent to visualize the redox states of protein by electrophoretic analysis. PEGPCMal has a maleimide group that can bind covalently to a protein thiol group. A mobility shift corresponding to about 5 kDa is observed by the electrophoretic analysis when one molecule of PEG-PCMal binds to a thiol group of the target protein. Thus, the number of free thiol groups on a protein can be clearly identified by SDS-PAGE through the mobility shift assay. The conventional reagents, PEG-Maleimide, have been widely used in mobility shift assays, however, transfer efficiency and antibody recognition on western blot are low due to the PEG chains labeled in protein. Because this reagent has a UV photocleavable moiety in the molecule, the PEG chains are cut off from the labeled protein in the gel with UV irradiation after the electrophoresis. Therefore, the protein treated with UV irradiation can be transferred from the gel to PVDF membrane and detected by antibodies.
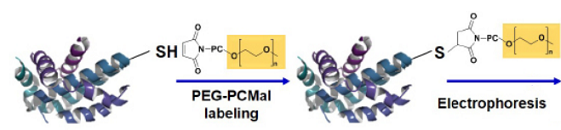
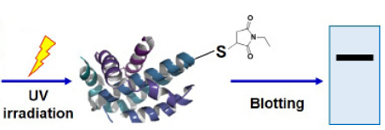
Fig. 1 Schematic protocol of PEG-PCMal
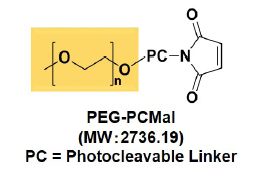
Fig. 2 Structure of PEG-PCMal
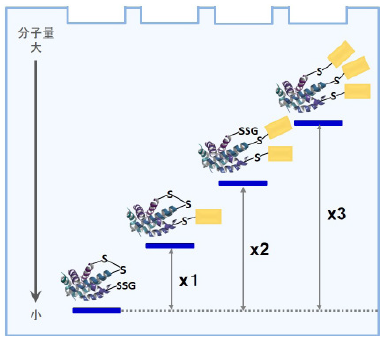
Fig. 3 Gel shift assay mechanism by PEG-PCMal depend on protein redox state
Content
| -SulfoBiotics- PEG-PCMal | 1 mg/tube |
Storage Condition
Store at 0-5 ℃ and protect from light.
Required Equipment and Materials
- 20 µl, 200 µl, 1000 µl pipettes
- Incubator (37 ℃)
- Centrifuge
- 1.5 ml-microtube
- Electrophoresis : Gel, Loading buffer, Staining reagent
- Western Blotting reagents: Transfer system, PVDF (polyvinyl difluoride) membrane, Transilluminator
Preparation
Dissolve in an appropriate buffer, water or DMSO depending on your experiment.
- 10 mmol/l PEG-PCMal DMSO solution is stable at -20 ℃ for up to a month. 10 mmol/l PEG-PCMal water solution is stable at -20 ℃ for up to two weeks.
General Protocol
-


Step 1
Add PEG-PCMal solution to a sample solution
containing proteins1)Step 2
Incubate at 37 °C 2)
(Labeling)Step 3
Gel electrophoresis3)
-


Step 4
Expose the gel in the glass plates with UV light
(Transilluminator)4)Step 5
Transfer the proteins to a membrane, and detect with an antibody.
Precautions
- Add PEG-PCMal solution to a sample so that the final concentration of PEG-PCMal become 1~5 mmol/l.
- Incubate at 37 ℃ for 30 min or at room temeperature for more than 1 hour. Proceed to Step 3 immediately after Step 2.
- Proceed to a gel staining after Step 3 for in-gel detection of proteins such as CBB staining.
- Expose the gel with UV light using a transilluminator (302 nm, 15W) for 10 minutes. Keep the gel in the glass plates to avoid drying it. In case of using a handy UV lamp (365 nm, 4W), expose the gel for 30 minutes. Do not use a handy UV lamp (254 nm) and a germicidal lamp.
Experimental Examples
Analysis of the redox states of TRX (Thioredoxin) in HeLa cells
-
- HeLa cells were seeded on a 96-well plate at the concentration of 1×105 cells/well and cultured overnight at 37 °C in a 5% CO2 incubator.
- The cells were washed with PBS (500 μl/well).
- PEG-PCMal (1 mg/tube) was dissolved with Lysis buffer (360 μl) to prepare 1 mmol/l PEG-PCMal.
- PEG-PCMal solution (1 mmol/l, 10 μl) was added to each well and mixed by pipetting to dissolve cells.
- The solution of Step 4 (10 μl) was transfered to a 1.5 ml-microtube, and incubated at 37 ℃ for 30 minutes.
- Loading buffer (2 μl) was added to the solution of Step 5 and mixed by pipetting.
*Loading buffer: 10(w/v)% sodium dodecyl sulfate (SDS), 50(v/v)% glycerol, 0.2 mol/l Tris-HCl (pH6.8), 0.05(w/v)% bromophenol blue. - The solution of Step 6 was used for SDS-polyacrylamide gel (10-20%) electrophoresis.
- The gel was exposed to UV rays (302 nm) using a transilluminator for 10 minutes.
- The separated proteins in the gel were electrophoretically transferred onto a PVDF membrane, and the proteins were detected with the antibody.
-
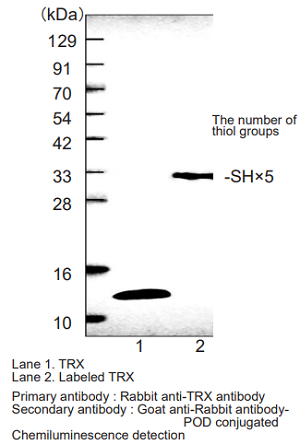
Fig.4 Visualization of the TRX redox state in HeLa cells
Analysis of the redox states of protein (ATP synthase γ subunit) in Arabidopsis thaliana
-
- A mortar and pestle were pre-cooled with liquid nitrogen for frost shattering.
- A piece of leaf of Arabidopsis thaliana (approx. 50 mg) was placed under dark or light condition for 5 minutes.
- The sample was placed on the pre-cooled mortar of Step 1 and freezed by liquid nitrogen. The sample was pulverized with a mortar and pestle.
- PEG-PCMal solution (4 mmol/l, 180 µl) was added to the sample (freezed instantly) and additionally pulverized with mortar and pestle.
- PEG-PCMal solution (4 mmol/l) was prepared by adding SDS sample buffer (90 µl) to PEG-PCMal (1mg/tube).
- *SDS sample buffer: 62.5 mmol/l Tris-HCl(pH7.5), 2% SDS, 7.5% glycerol, 0.01% bromophenol blue.
- The pulverized sample was transffered to a 1.5 ml-microtube and incubated at r.t. for 1 hour under dark condition.
- The reaction mixture of Step 4 was boiled at 95 ℃ for 5 minutes and centrifuged at 15,000 rpm for 10 minutes.
- The supernatant of Step 5 was used as a sample for electrophoresis.
- The gel was exposed to UV rays (302 nm) using a transilluminator for 10 minutes.
- The separated proteins in the gel were electrophoretically transferred onto a PVDF membrane, and the proteins were detected with the antibody.
-
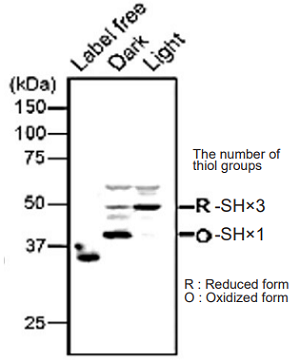
Fig. 5 Visualization of the redox state of ATP synthase γ subunit in Arabidopsis thaliana
Technical advisors
Dr. Toru Hisabori (Laboratory for Chemistry and Life Science, Institute of Innovative Research,Tokyo Institute of Technology)
Dr. Keisuke Yoshida (Laboratory for Chemistry and Life Science, Institute of Innovative Research,Tokyo Institute of Technology)
Dr. Satoshi Hara (School of Life Science and Technology, Tokyo Institute of Technology)
Frequently Asked Questions / Reference
SB20: -SulfoBiotics- PEG-PCMal
Revised Aug., 22, 2023


 Hidden sections will not be printed.
Hidden sections will not be printed.

