General Information
Modification of protein thiols is one of the most important post-translational modifications and it occurs according to the redox states in cells. It has recently been revealed that the modifications of thiol groups control cellular functions such as transcription, protein expression, cell death, etc. Therefore, detection of the redox states of the individual protein is important to understand cellular events.
-SulfoBiotics- Protein Redox State Monitoring Kit Plus enables visualization of the redox states of protein by electrophoretic analysis. Protein-SHifter Plus, one of the components in the kit, has a maleimide group that can bind covalently to a protein thiol group. A mobility shift corresponding to about 15 kDa of molecular mass is observed by the electrophoretic analysis when one molecule of Protein-SHifter Plus binds to a thiol group of the target protein. Thus, the number of free thiol groups on a protein can be clearly identified by SDS-PAGE through the mobility shift assay. In addition, the Protein-SHifter moiety is cut off from the labeled protein in the gel with UV irradiation after the electrophoresis, since Protein-SHifter Plus has a UV photocleavable moiety in the molecule. Therefore, the protein treated with UV irradiation can be transferred from the gel to PVDF membrane and detected on the membrane similar to the unlabeled protein.
*This protocol in this manual is optimized for mammalian cells.
- This protocol in this manual is optimized for mammalian cells.
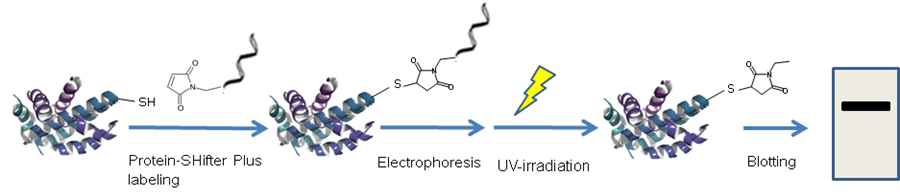
Figure 1 Schematic protocol of Protein Redox State Monitoring Kit Plus
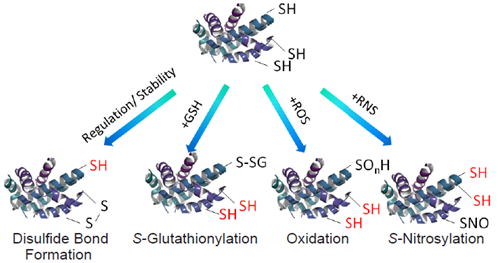
Figure 2 Representative protein thiol modifications
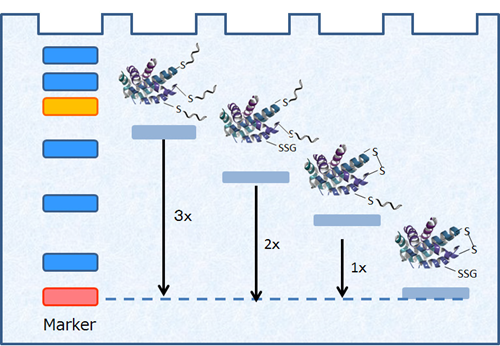
Figure 3 Visualization of protein redox states
A mobility shift corresponding to about 15 kDa is observed when one molecule of Protein-SHifter Plus binds to a thiol group of a protein.
Kit Contents
| 20 samples | |
| Lysis Buffer | x 20 |
| Protein-SHifter Plus | x 4 |
| Reaction Buffer A | x 4 |
| Reaction Buffer B | x 4 |
Storage Condition
Store at 0 - 5°C
Required Equipment and Materials
-
- 10 μl and 100-200 μl pipettes
- Incubator (37°C)
- Electrophoresis: Gel, Loading Buffer
- Centrifuge
- Westernblot: Transfer system, PVDF(polyvinyl difluoride) membrane
- Transilluminator
-
- 10 (w/v) % TCA (Trichloroacetic acid) solution
- Acetone
- 70 (v/v) % EtOH solution
- PBS
- Microtube (1.5 ml)
Precaution
- Centrifuge the tube containing Protein-SHifter Plus briefly before opening the cap because the content may be on the tube wall or on the ceiling of the cap.
- If a precipitate is observed in Reaction Buffer B, warm at 40-50℃ to dissolve it.
- This kit is optimized for mammalian cells.
Sample preparation
For adherent cells
- Prepare 5 - 10 x 105 cells/well in 6-well culture plate for the assay.
- The concentration of proteins and thiols should be as follows; proteins: 0.1 - 1 mg/ml, thiols: < 100 μmol/l.
- Visit our website (SB12 Q&A) or contact our technical support for information about the way to determine the concentration of thiols in your sample.
- Aspirate the medium and wash the cells twice with cold 500 μl PBS.
- Add 500 μl of cold 10 % TCA solution to each well and incubate the plate on ice for 30 minutes.
- This step is for protecting thiol groups of the protein from oxidation and removing thiol chemicals such as glutathione from the sample.
- Recover the cells from the wells and transfer them to tubes.
- Centrifuge at 1,000 x g for 3 minutes and remove the supernatant.
- Add 500 μl of cold acetone to each tube, centrifuge at 1,000 x g for 3 minutes and remove the supernatant.
- Repeat Step 6.
- Add 500 μl of cold 70% EtOH solution to each tube, centrifuge at 1,000 x g for 3 minutes and remove the supernatant.
- Add 100 μl of Lysis Buffer (Add protease inhibitor, when needed.) to each tube and dissolve the cell pellet by sonication.
- If the gelation of the solution occurs, dilute it with Lysis Buffer.
- Proceed to the following General Protocol immediately.
For suspension cells
- Prepare 5 - 10 x 105 cells/tube for the assay.
- The concentration of proteins and thiols should be as follows; proteins: 0.1 - 1 mg/ml, thiols: < 100 μmol/l.
- Visit our website (SB12 Q&A) or contact our technical support for information about the way to determine the concentration of thiols in your sample.
- Centrifuge at 1,000 x g for 3 minutes and remove the supernatant.
- Add 500 μl of PBS to each tube, centrifuge at 1,000 x g for 3 minutes and remove the supernatant .
- Repeat step 3.
- Add 500 μl of cold 10 % TCA solution to each tube and incubate the tube on ice for 30 minutes.
- This step is for protecting thiol groups of the protein from oxidation and removing thiol chemicals such as glutathione from the sample.
- Centrifuge at 1,000 x g for 3 minutes and remove the supernatant.
- Add 500 μl of cold acetone, centrifuge at 1,000 x g for 3 minutes and remove the supernatant.
- Repeat Step 7.
- Add 500 μl of cold 70% EtOH solution, centrifuge at 1,000 x g for 3 minutes and remove the supernatant.
- Add 100 μl of Lysis Buffer (Add protease inhibitor, when needed.) and dissolve the cell pellet by sonication.
- If the gelation of the solution occurs, dilute it with Lysis Buffer.
- Proceed to the following General Protocol immediately.
General Protocol
- Add 4 μl of Reaction Buffer A to a tube of Protein-SHifter Plus.
- Protein-SHifter Plus is unstable in Reaction Buffer A,B. Proceed to Step 2 immediately after adding Reaction Buffer A.
- Add 2 μl of the sample solution to the solution of Step 1 and mix by pipetting.
- Add 4 μl of Reaction Buffer B to the solution of Step 2 and mix by pipetting.
- Warm the solution to completely dissolve a precipitate if precipitation occurs.
- Defoam by centrifugation at 7,000 x g for 1 - 2 minutes if foaming occurs.
- Incubate at 37°C for 30 minutes.
- Proceed to Step 5 immediately.
- Add a Loading buffer to the solution of Step 4 and apply the solution to gel electrophoresis.
- This kit does not contain Loading buffer. When using (5x) Loading buffer, add 2 μl of (5x) Loading buffer to 10 μl of the solution of Step 4 and apply all of the solution to the gel.
- Expose the gel to UV rays (302 nm) with a transilluminator for 10 minutes.
- During this step, keep the gel in the glass plates to avoid drying it.
- Transfer proteins from the gel to PVDF membrane.
- Detect a target protein with a specific antibody.
Usage Example
Redox response of GAPDH (Glyceraldehyde 3-phosphate dehydrogenase) to oxidants in HeLa cells.
- HeLa cells were seeded on a 6-well plate at the concentration of 5 x 105 cells/well and cultured overnight at 37 °C in a 5% CO2 incubator.
- The cells were washed using cold 500 μl PBS, and Diamide [1,1’-Azobis(N,N-dimethylformamide)] or H2O2 (1 mmol/l, 500 μl) were added to the culture cells.
- The cells were then incubated at 37 °C for 15 minutes.
- Following aspiration of the supernatant, cold TCA solution (10 %, 500 μl) was added to each well.
- The plate was incubated on ice for 30 minutes.
- The cells were scraped and transferred to microtubes.
- After the tubes were centrifuged at 1,000 x g for 3 minutes, the supernatants were removed out.
- Cold acetone (500 μl) was added to each tube, and the supernatants were removed out after centrifugation of the tubes at 1,000 x g for 3 minutes.
- Step 8 was repeated.
- Cold 70% EtOH solution (500 μl) was added, and the supernatants were removed out after centrifugation of the tubes at 1,000 x g for 3 minutes.
- Lysis Buffer containing 1% protease inhibitors (100 μl) was added, and the tubes were sonicated to dissolve the cell pellets completely.
- Reaction Buffer A (4 μl) was added to a tube including Protein-SHifter Plus and mixed by pipetting.
- The sample solution of Step 11 (2 μl) was added to each tube of Step 12 and mixed by pipetting.
- Reaction Buffer B (4 μl) was added to each tube of Step 13 and mixed by pipetting.
- The tube of the Step 14 was incubated at 37 °C for 30 minutes.
- Loading Buffer ([10 (w/v)% sodium dodecyl sulfate, 50(v/v)% glycerol, 0.2 mol/l Tris-HCl (pH 6.8) , 0.05(w/v)% bromophenol blue], 2 μl) was added to the tube at Step 15 and was mixed by pipetting.
- The solution of Step 16 was used for SDS-polyacrylamide gel (15%) electrophoresis.
- The gel was exposed to UV rays (302 nm) using a transilluminator for 10 minutes.
- The separated proteins in the gel were electrophoretically transferred onto a PVDF membrane.
- The GAPDH on the membrane was detected with anti-GAPDH antibody.
- Lane 1: no-oxidants
Lane 2: Diamide oxidation
Lane 3: H2O2 oxidation
Primary antibody: Rabbit anti-GAPDH antibody
Secondary antibody:Goat anti-Rabbit antibody-POD conjugated
15% SDS-polyacrylamidegel, chemiluminescence detection
Figure 4 Redox states of GAPDH treated with oxidants in HeLa cells
Reference
- Satoshi Hara, Tatsuya Nojima, Kohji Seio, Masasuke Yoshida, Toru Hisabori, "DNA-maleimide: An improved maleimide compound for electrophoresis-based titration of reactive thiols in a specific protein" Biochim. Biophys. Acta, 2013, 1830(4) 3077.
- Satoshi Hara, Yuki Tatenaka, Yuya Ohuchi, Toru Hisabori, "Direct determination of the redox status of cysteine residues in proteins in vivo", Biochem. Biophys. Res. Commun., 2015, 456(1) 339.
Frequently Asked Questions / Reference
SB12: -SulfoBiotics- Protein Redox State Monitoring Kit Plus
Revised Apr., 05, 2024


 Hidden sections will not be printed.
Hidden sections will not be printed.


