General Information
It becomes obvious that there are a lot of molecules containing sulfane sulfurs such as persulfides and polysulfi des in living body. These molecular species are involved in production, storage and release of hydrogen sulfide, which is recognized as an important physiological mediator. Furthermore, recent studies reveal that persulfides and polysulfides may control intracellular signal transduction through s-sulfhydration of proteins, and function in vivo as anti-oxidants which have much higher reducing activity than glutathione reduced form and cysteine.
Sodium polysulfi des (Na2Sn) are sulfane sulfur donors which have simple structures, and exist as hydrogen polysulfi des in an aqueous solution depending on pH. These reagents are necessary to for research and analysis of sulfane sulfurs in vivo.
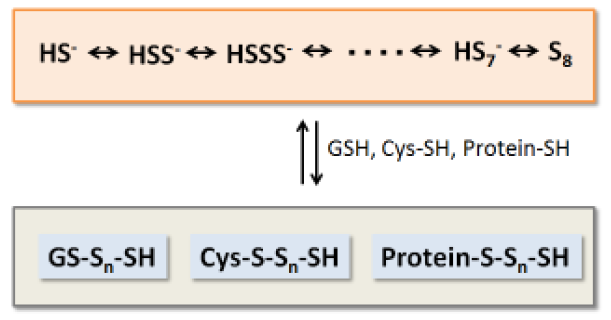
Fig. 1 Chemical species containing sulfane sulfurs
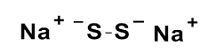
Sodium disulfi de (Na2S2)
pKa1 = 5
pKa2 = 9.7
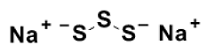
Sodium disulfi de (Na2S3)
pKa1 = 4.2
pKa2 = 7.5
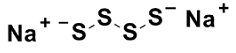
Sodium disulfi de (Na2S4)
pKa1 = 3.8
pKa2 = 6.3
※The pKa values were refered to the following article.
J. Gun et al., “Electrospray Ionization Mass Spectrometric Analysis of Aqueous Polysulfi de Solutions”,
Microchim. Acta, 2004, 146, 229
Fig. 2 Structures of Sodium polysulfi des (Na2Sn) and the pKa values
Contents
| SB02 | -SulfoBiotics- Sodium disulfide (Na2S2) | : 100 mg x 5 |
| SB03 | -SulfoBiotics- Sodium trisulfide (Na2S3) | : 100 mg x 5 |
| SB04 | -SulfoBiotics- Sodium tetrasulfide (Na2S4) | : 100 mg x 5 |
| SB13 | -SulfoBiotics- Sodium Polysulfide Set | : Na2S2, Na2S3, Na2S4 100 mg each |
Storage Condition
Store at 0-5°C
- Open the cap after reaching to room temperature because they are moisture sensitive.
Store at 0-5°C under nitrogen gas, and use up the reagent early after opening.
Experimental Example 1
- 10 μl of 10 mmol/l Na2Sn aqueous solution was added to 1 ml of 20 μmol/l WST-8 (PBS) solution.
- The solution was incubated at room temperature for 30 minutes, transfer 100 μl of the solution to each well, and measure the absorbance at 450 nm using a microplate reader.
- WST-8 is a highly water-soluble tetrazolium salt developed by Dojindo Laboratories, and gives a yellowcolor formazan dye by reduction.
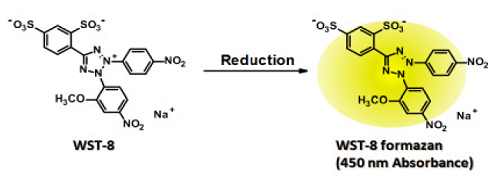
Fig. 4 Reduction of WST-8
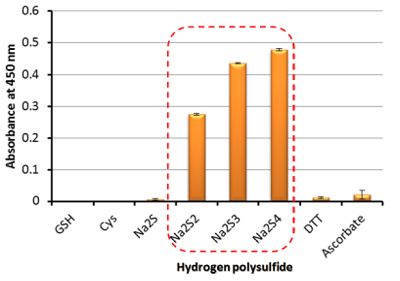
Fig. 5 Absorbances at 450 nm of WST-8 formazans produced with various reducing agents.
Hydrogen polysulfi des gave high absorbances at 450 nm derived from WST-8 formazans. On the other hand, no absorbances at 450 nm were observed when glutathione reduced form (GSH) and cysteine (Cys) were used as reducing agents. These results show that hydrogen polysulfi des have much higher reducing activity than the general reducing agents.
Experimental Example 2
Detection of sulfane sulfurs in cells treated with a sulfane sulfur donor (Na2S3)
- CHO cell suspensions prepared with serum-containing DMEM were inoculated in a 96-well black clear bottom plate to prepare 104 cells/well, and incubated in a humidifi ed incubator (e.g., at 37℃, 5% CO2) overnight.
- The culture medium was discarded, and the cells were washed with a serum-free DMEM.
- 100 μl of a 100 μmol/l Na2S3 (serum-free DMEM) was added to the each well, and the cells were incubated for 15 minutes in the incubator.
- The supernatant was discarded, and the cells were washed with a serum-free DMEM twice.
- 100 μl of a 20 μmol/l SSP4 (serum-free DMEM) was added to the cells, and the cells were incubated for 15 minutes in the incubator.
- The supernatant was discarded, and the cells were washed with PBS twice.
- 100 μl of PBS was added to the each wells, and the cells were analyzed under a fluorescence microscope.
- SSP4 (SB10; -SulfoBiotics- SSP4) is a novel fluorescent probe to detect sulfane sulfurs selectively.
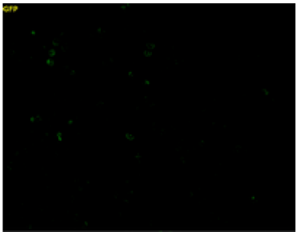 Control Cells |
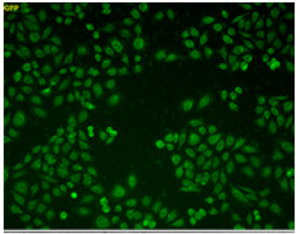 100 μmol/l Na2S3 treated Cell |
(Exporsue time: 1000 msec) |
| Fluorescence signals were observed in cells treated with 100 μmol/l Na2S3 (sulfane sulfur donor). Fig. 6 Fluorescence images of sulfane sulfurs in cells treated with or without Na2S3 |
References
- Y. Kimura, Y. Mikami, K. Osumi, M. Tsugane, J. Oka, and H. Kimura, FASEB J., 2013, 27, 2451.
- S. Koike, Y. Ogasawara, N. Shibuya, H. Kimura, and K. Ishii, FEBS Lett., 2013, 587, 3548.
- T. Ida, T. Sawa, H. Ihara. Y. Tsuchiya, Y. Watanabe, Y. Kumagai, M. Suematsu, H. Motohashi, S. Fujii, T. Matsunaga, M.Yamamoto, K. Ono, N. O. Devarie-Baez, M. Xian, J. M Fukuto, and T. Akaike, Proc. Natl. Acad. Sci. USA., 2014, 111, 7606.
SB02_SB03_SB04_SB13: -SulfoBiotics- Sodium disulfide (Na2S2) / (Na2S3) / (Na2S4) / Sodium Polysulfide Set
Revised Apr., 05, 2024


 Hidden sections will not be printed.
Hidden sections will not be printed.