General Information
It has been recognized that hydrogen sulfide (H2S) has an important role as a physiological active substance for vasodilation, cytoprotection, and modulation of insulin secretion. H2S is considered as a gaseous molecule such as NO and CO. However, around 80% of the total sulfide exists as hydrogen sulfide anion (HS-) under physiological condition, since the pKa is about 7 (Fig. 1). In addition, H2S easily converts to various biochemical molecules such as persulfides and polysulfides, which react with sulfhydryl moieties in a living body. The functional mechanism of H2S has not been well understood.Sodium sulfide (Na2S) has been widely used as a H2S donor. Na2S is readly decomposed and release H2S when Na2S is dissolved in H2O.
Fig. 1 Physiological functions of hydrogen sulfide
Contents
| -SulfoBiotics- Sodium sulfide (Na2S) | 100 mg x 5 |
Storage Condition
Store at 0-5 °C
Precaution
- Use the reagent after bringing to room temperature, because it is moisture sensitive.
- Use up the reagent soon after opening.
General Protocol
- Dissolve 7.8 mg of Na2S in 1 ml of deionized H2O under nitrogen gas (100 mmol/l Na2S solution).
- Dilute the 100 mmol/l Na2S aqueous solution to an appropriate concentration depending on your experiment.
- Purge deionized H2O with nitrogen gas for longer than 30 minutes to prevent the oxidation.
- Use the aqueous solution as soon as prepared. The solution is not stable enough to store.
Experimental Example
- Detection of hydrogen sulfide by methylene blue method -
- 20 µl of 100 mmol/l Na2S aqueous solution was added to 980 µl of deionized H2O to prepare 2 mmol/l Na2S solution.
- 100 μl of 2 mmol/l Na2S solution was added to 900 µl of deionized H2O to prepare 200 µmol/l Na2S solution.
- 200 µmol/l Na2S solution was diluted with deionized H2O to prepare various concentration of Na2S solution by serial dilution (200, 100, 50, 25, 12.5, 6.3, 3.2, 0 µmol/l).
- 300 μl of 1% zinc acetate solution , 50 μl of 20 mmol/l N,N-Dimethyl-p-phenylenediammonium (7.2 mol/l HCl) solution and 50 μl of 30 mmol/l FeCl3 (1.2 mol/l HCl) solution were added to 250 μl of the Na2S solutions and mixed using a vortex.
- The solutions were incubated at room temperature for 30 minutes and transfered 200 µl of the solution to each well (96-well plate).
- Measure the absorbance at 650 nm using a microplate reader (Fig. 2).
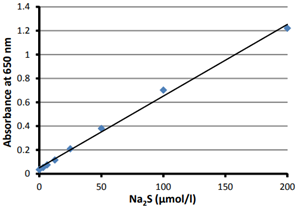
Fig. 2 Absorbance change at 650 nm depending on the concentration of hydrogen sulfide
References
- R. Greiner, Z. Palinkas, K. Basell, D. Becher, H. Antelmann, P. Nagy and T. P. Dick, “Polysulfides link H2S to protein thiol oxidation”, Antioxid. Redox Signal., 2013, 19, 1749.
- N. S. Lawrence, J. Davis and R. G. Compton, “Analytical strategies for the detection of sulfide: a review”, Talanta, 2000, 52, 771.
Frequently Asked Questions / Reference
SB01: -SulfoBiotics- Sodium sulfide (Na2S)
Revised Nov., 21, 2023


 Hidden sections will not be printed.
Hidden sections will not be printed.