General Information
Sperm, which are germ cells, contain protamines, which replace histones and bind nuclear proteins. Protamines form higher-order structures through disulfide bonds. As a result, compared with somatic cell nuclei, sperm nuclei have highly condensed chromatin structures.
This kit reduces the disulfide bonds that are abundant in protamines. The resulting reduced thiol groups then bind a thiol-reactive fluorescent dye (Thiol-specific Dye Blue). Simultaneously, the DNA is stained with the fluorescent dye Acridine Orange (AO). When the Thiol-specific Dye Blue is excited with ultraviolet (UV) light, the resulting fluorescence energy is transferred to the AO bound to the nearby DNA through fluorescence resonance energy transfer (FRET), causing the sperm head to emit strong green fluorescence.
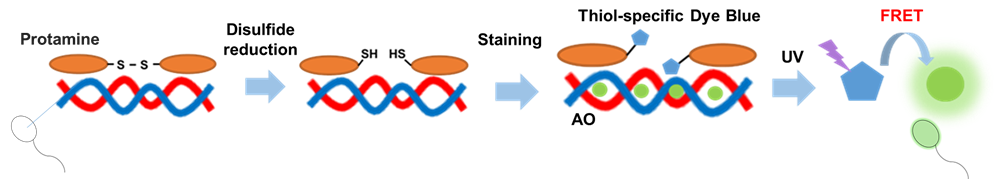
Fig. 1 Principles of staining of sperm protamine–DNA complexes
Fluorescent Property
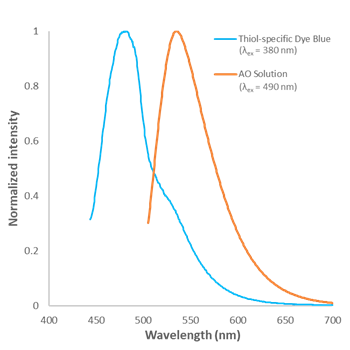
Fig. 2 Fluorescence spectra of Thiol-specific Dye Blue and AO in Assay Buffer
Kit Contents
| Thiol-specific Dye Blue | 10 µl × 1 |
| AO Solution | 75 µl × 1 |
| Reducing Agent | × 1 |
| Assay Buffer | 40 ml × 1 |
Storage Condition
Store at -20 °C and protect from light.
Required Equipment and Materials
- 100-1,000 µl, 20-200 µl, 2-20 µl, and 0.1-2 µl micropipettes
- Conical tubes
- Proteinase K solution
- 4% Paraformaldehyde (PFA) solution in phosphate-buffered saline (PBS)
- Slide glass, Cover glass
- Antifade mounting media for fixed cells
Preparation of Solutions
Preparation of Reducing Agent (RA) stock solution
Add 100 μl of ddH2O to the provided tube containing Reducing Agent, and dissolve by pipetting.
- Store the RA stock solution at -20°C and protect it from light. The RA solution is stable for 6 months.
- If precipitate remains after freezing/thawing, dissolve by vortexing.
General Protocol
- Gently smear semen or the swab extract onto a slide glass. (6 mm spot)
- The samples should be allowed to dry thoroughly.
- If contaminants are in the sample, pre-treatment using Proteinase K improves sperm visibility.
- Please refer to the Q&A on the product web page for details of sample preparation and Proteinase K treatment.
- Add 4% PFA solution in PBS to the samples and incubate at room temperature for 5 min.
- Discard the PFA/PBS solution and wash the samples twice with Assay Buffer (30 µl).
- Prepare enough RA working solution for samples.
Slide glass, 30 µl RA working solution/test 1 test 10 tests 100 tests RA stock solution 0.8 μl 8 μl 80 μl Assay Buffer 33 μl 330 μl 3,300 μl Total 33.8 μl 338 μl 3,380 μl - RA working solution should be prepared immediately before use.
- RA working solution cannot be stored. The RA working solution should be used up on the day it is prepared.
- Add RA working solution (30 µl) and incubate at room temperature for 5 min.
- Discard the supernatant and wash the samples twice with Assay Buffer (30 µl).
- Prepare enough Staining solution for samples.
Slide glass, 30 µl Staining solution/test 1 test 10 tests 100 tests Thiol-specific Dye Blue 0.1 μl 1 μl 10 μl AO Solution 0.1 μl 1 μl 10 μl Assay Buffer 33 μl 330 μl 3,300 μl Total 33.2 μl 332 μl 3,320 μl - Staining solution should be prepared immediately before use.
- Staining solution cannot be stored. The Staining solution should be used up on the day it is prepared.
- Add Staining solution (30 µl) and incubate at room temperature for 5 min.
- Discard the supernatant and wash the samples twice with Assay Buffer (30 µl).
- Add antifade mounting media, and gently seal the slide with a cover glass.
- Observe the samples under a fluorescence microscope.
Usage Example
Fluorescence imaging of semen and buccal cells by confocal fl uorescence microscopy
- Proteinase K-treated semen and oral cells (untreated) were prepared and placed onto glass slides (6 mm spot),respectively.
- 4% PFA solution in PBS (30 μl) was added to the samples, which were incubated at room temperature for 5 min.
- The supernatant was discarded, and the samples were washed twice with Assay Buff er (30 μl).
- RA working solution (30 μl) was added to the samples, and the mixture was incubated at room temperature for 5 min.
- The supernatant was discarded, and the samples were washed twice with Assay Buff er (30 μl).
- Staining solution (30 μl) was added to the samples, and the mixture was incubated at room temperature for 5 min.
- The supernatant was discarded, and the samples were washed twice with Assay Buff er (30 μl).
- Antifade mounting media (ProLong™ Glass Antifade Mountant, Thermo; 1 drop) was added to the samples, whichwere gently covered with a cover glass to seal the slide.
- The samples were observed by confocal fl uorescence microscopy (LSM 800, Zeiss; x20).
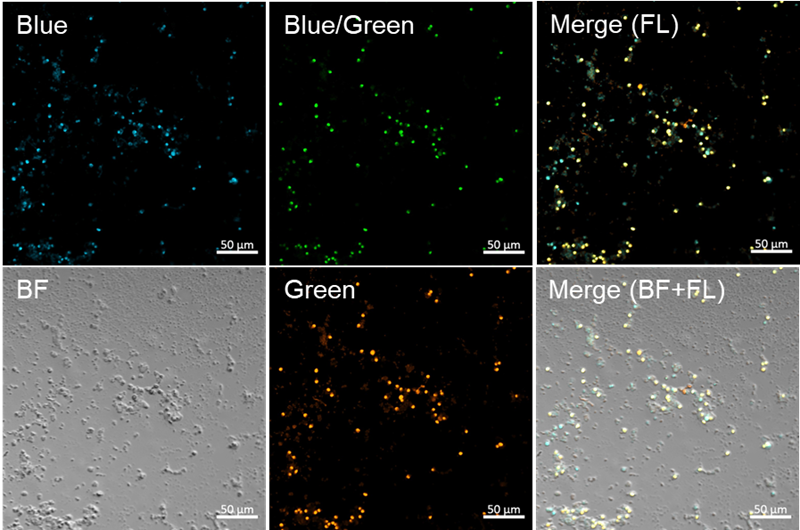
Fig. 3 Fluorescence imaging of semen
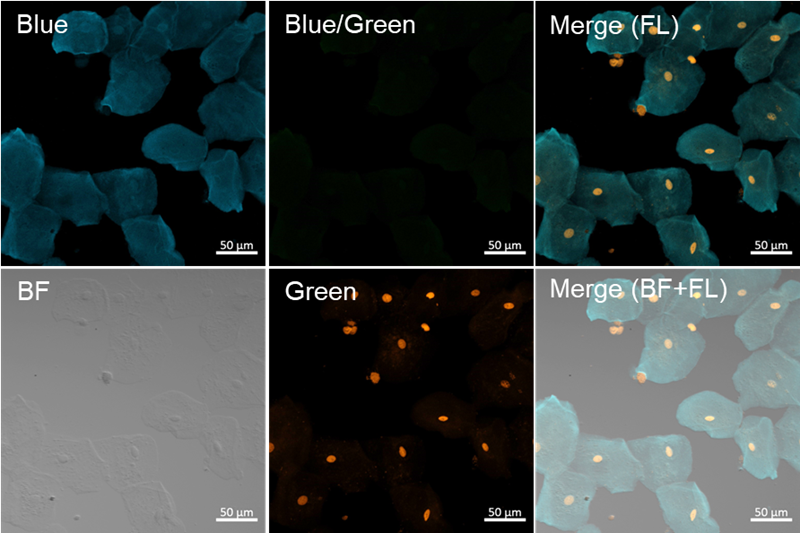
Fig. 4 Fluorescence imaging of buccal cells
<Experimental Conditions>
- Blue filter sets: Ex = 405 nm, Em = 450–510 nm, Gain: 0.15%, 550V
- Blue/Green filter sets: Ex = 405 nm, Em = 520–530 nm, Gain: 0.15%, 550V
- Green filter sets: Ex = 488 nm, Em = 520–530 nm, Gain: 0.10%, 550V
S395: Sperm Staining Kit
Revised Oct., 23, 2024


 Hidden sections will not be printed.
Hidden sections will not be printed.