General Information
A Nucleolus is one of the structures forming a nucleus and becoming the starting point of the ribosomal biogenesis. There are many ribosomal RNA (rRNA) in the nucleolus and transcription of rRNA and processing are carried out. Whereas the morphological change of nucleolus is known as one of the indicator of cancer diagnosis, there has been some scientific reports describing the relation between nucleolus and DNA damge1), autophagy2), virus infection3), and cellular senescence4), 5). Nucleolus Bright dyes are small molecules and they bind to RNA in the nucleolus to emit fluorescnece. The nucleolus can be observed without any washing steps after staining with Nucleolus Bright dyes.
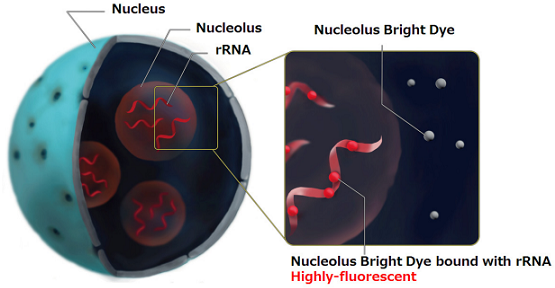
Figure 1. Staining system of Nucleolus Bright
Contents
| N511 Nucleolus Bright Green | 60 nmol x 1 |
| N512 Nucleolus Bright Red | 60 nmol x 1 |
- Equivalent to 30 tests when 35 mm dish is used. (final concentration of each dye: 1 µmol/l)
Storage Condition
| N511 Nucleolus Bright Green | Store in a cool and dark place, protect from light. |
| N512 Nucleolus Bright Red | N512 Store in a cool and dark place |
Required Equipment and Materials
- Dimethyl sulfoxide (DMSO)
- PBS
- Micropipettes
Fluorescent property
Fluorescent property of Nucleolus Bright
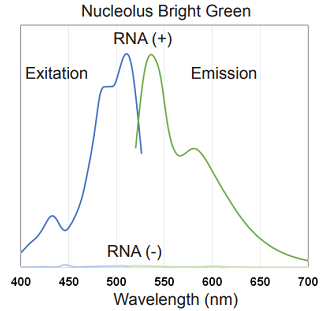
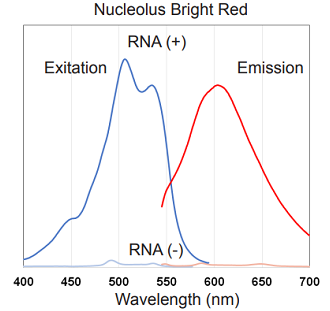
Figure 2. Excitation and emission spectra of Nucleolus Bright Green and Red
Preparation of Solutions
Preparation of 1 mmol/l of Nucleolus Bright DMSO stock solution
Add 60 µl of DMSO to a tube of Nucleolus Bright and dissolve by pipetting.
- Store the DMSO stock solution at -20 oC. The DMSO stock solution is stable at -20 oC for 1 month.
Preparation of Nucleolus Bright working solution
Dilute the DMSO stock solution with PBS to prepare 0.5–5 µmol/l working solution.
- Use the working solution within the same day of preparation.
General Protocol
Nucleolus Bright staining
- Seed cells on a dish for assay. Culture the cells at 37 oC overnight in a 5% CO2 incubator.
- Remove the culture medium and fix the cells by adding 4% paraformaldehyde (PFA) /PBS solution or cool methanol to each well and incubate at room temperature.
- Incubation time 4% PFA: 5 minutes, cool methanol: 1 minute
- Remove the supernatant and wash the cells with PBS three times.
- Add 1% Triton X-100/PBS solution to each well and incubate at room temperature for 20 minutes.
- Remove the supernatante and wash the cells with PBS three times.
- Add Nucleolus Bright working solution and incubate at room temperature for 5 minutes.
- Observe the sample under a fluorescence microscope.
Usage samples
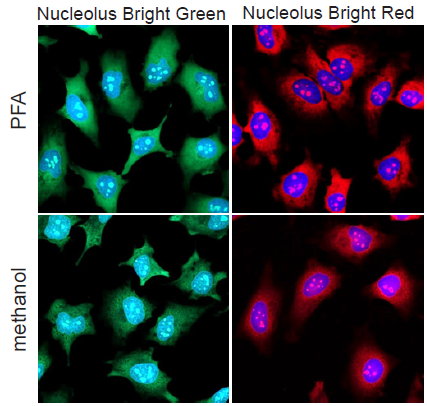
Fluorescence imaging of nucleoli in HeLa cells
- HeLa cells were seeded on a μ-slide 8 well plate and cultured at 37 ℃ overnight in a 5% CO2 incubator.
- After the culture medium was removed, 4% PFA/PBS solution or cool methanol was added to each well and the plate was incubated at room temperature.
- Incubation time, 4% PFA: 5 minutes, cool methanol: 1 minute.
- The supernatant was removed and the cells were washed with PBS three times.
- To permeabilize the nuclear membrane, 1% Triton X-100/PBS solution was added to each wells and incubated at room temperature for 20 minutes.
- The supernatant was removed and the cells were washed with PBS three times.
- The mixture of Nucleolus Bright working solution and DAPI solution (200 µl) was added and the cells was incubated at room temperature for 5 minutes.
- The cells were observed under a fluorescence microscope.
-
Nucleolus Bright Green
(Dye concentration: 1 μmol/l)
(Ex: 488 nm, Em: 500–600 nm)
Nucleolus Bright Red
(Dye concentration: 1 μmol/l)
(Ex: 561 nm, Em: 565–650 nm)
DAPI
(Dye concentration: 3.6 μmol/l)
(Ex: 405 nm, Em: 450–495 nm)
Figure 3. Fluorescence imaging of nucleoli in HeLa cells
(green: Nucleolus Bright Green, red: Nucleolus Bright Red, blue: DAPI)
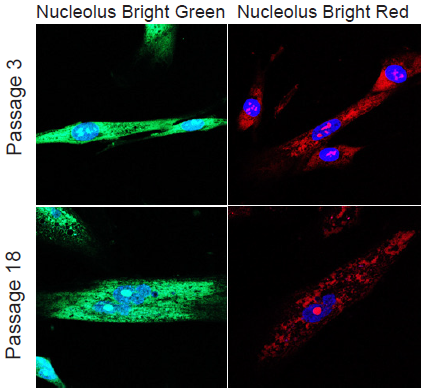
Morphological change of nucleoli depending on cellular senescence
- WI-38 cells (5×104 cells/dish, MEM, 10% fetal bovine serum, 1% penicillin-streptmycin) of different passage number were seeded on a μ-slide 8 well plate and cultured at 37 ℃ overnight in a 5% CO2 incubator.
- After the culture medium was removed, 4% PFA/PBS solution was added to each well and the plate was incubated at room temperature for 5 minutes.
- The supernatant was removed and the cells were washed with PBS three times.
- To permeabilize the nuclear membrane, 1% Triton X-100/PBS solution was added to each wells and incubated at roomtemperature for 20 minutes.
- The supernatant was removed and the cells were washed with PBS three times.
- The mixture solution of Nucleolus Bright working solution and DAPI solution (200 µl) was added and the cells was incubated at room temperature for 5 minutes.
- The cells were observed under a fluorescence microscope.
-
Nucleolus Bright Green
(Dye concentration: 1 μmol/l)
(Ex: 488 nm, Em: 500–600 nm)
Nucleolus Bright Red
(Dye concentration: 1 μmol/l)
(Ex: 561 nm, Em: 565–650 nm)
DAPI
(Dye concentration: 3.6 μmol/l)
(Ex: 405 nm, Em: 450–495 nm)
Figure 4. Morphological change of nucleoli dependimg on cellular senescence in WI-38 cells
(green: Nucleolus Bright Green, red: Nucleolus Bright Red, blue: DAPI)
Reference
- Greenberg, R. A. et al., Cell Reports, 2015, 13, 251–259.
- Kimura, K. et al., Scientific Reports, 2015, 5, 8903.
- Hiscox, J. A. et al., Cellular Microbiology, 2006, 8, 1147–1157.
- Nakao, M. et al., Cell Reports, 2017, 18, 2148–2161.
- Antebi, A. et al., Trends in Cell Biology, DOI: 10.1016/j.tcb.2018.03.007.
This product was developed under the advisory of Dr. Mitsuyoshi Nakao (Kumamoto University)
N511_N512: Nucleolus Bright Green/ Red
Revised Jun., 26, 2023


 Hidden sections will not be printed.
Hidden sections will not be printed.