General Information
Nicotinamide adenine dinucleotide (NAD) is an important cofactor involved in redox reactions in the main metabolic pathways in cells such as glycolysis, electron transfer system and TCA cycle. NAD exists as an oxidized form NAD+ and a reduced form NADH in cells. Maintaining appropriate NAD+ and NADH levels is essential for cell function. In addition, recent study suggests that decrease of NAD+ levels is related to senescence1), and the amount of NAD+ is thought to be a marker in senescence-related studies.
NAD/NADH Assay Kit-WST enables quantitation of the amount of total NAD+/NADH, NADH and NAD+ in cells and measurement of their ratio. The intracellular NADH levels can be quantitated selectively by heat treatment of cell lysate with the extraction buffer in this kit. The intracellular NAD+ levels can also be determined by subtracting the amount of NADH assayed from the amount of total NAD+/NADH.
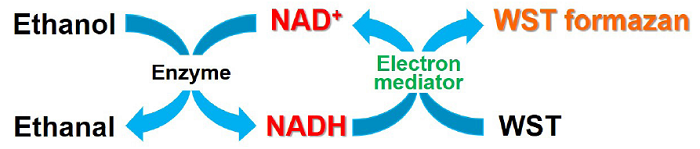
Fig. 1 Principle of NAD measurement by NAD/NADH Assay Kit-WST
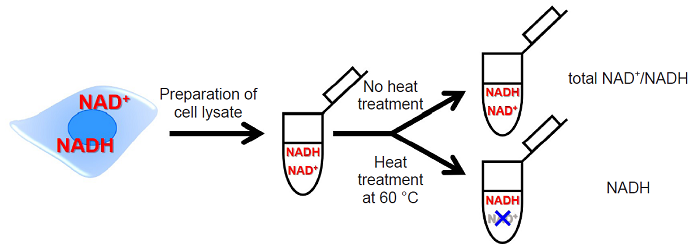
Fig. 2 Detection method of total NAD+/NADH, NADH and NAD+
Kit Contents
| NAD/NADH Extraction Buffer | 20 mL x 1 |
| NAD/NADH Control Buffer | 20 mL x 1 |
| Standard Buffer | 10 mL x 1 |
| Assay Buffer | 5.5 mL x 1 |
| Dye Mixture(Red cap) | x 1 |
| Enzyme(Green cap) | x 1 |
| Standard(Blue cap) | x 1 |
| Filtration Tube | x 12 |
- The filtration tubes (12×) are sufficient for measuring 12 test samples. For additional samples, commercially available filtration tubes (e.g., Nanosep Centrifugal Devices (10K)([OD010C33], PALL)) is recommended.
Storage Condition
Store at 0-5 °C
Required Equipment
- Microplate reader (450 nm filter)
- 96-well microplate
- Incubator (37 °C, 60 °C)
- 20-200 μL multichannel pipette
- 100-1000 μL, 20-200 μL, 2-20 μL micropipettes
Precautions
- Equilibrate the kit to room temperature prior to use.
- Centrifuge the tube (Enzyme and Standard) briefly before opening the cap because the content may be on the tube wall or on the ceiling of the cap.
- Analyzing samples and standards in triplicate is recommended for accuracy.
- Since the enzymatic reaction starts immediately after the addition of Working solution to a well, use a multichannel pipette to minimize the experimental error from time lag in pipetting.
- Please prepare samples with different dilution rate and determine a suitable dilution rate to be ranging from 0 to 2μmol/L.
Preparation of Solutions
Preparation of Enzyme stock solution
Add 35 μL of PBS to a Enzyme tube and dissolve it with pipetting.
- Centrifuge the tube briefly before opening the cap because the content may be on the tube wall or on the ceiling of the cap.
- Enzyme stock solution should be kept in ice bath during your experiment.
- Store the Enzyme stock solution at 0-5 °C. The solution is stable at 0-5 °C for 2 months.
Preparation of Standard stock solution (10 mmol/L)
Add 20 μL of double-deionized H2O (ddH2O) to a tube of Standard and dissolve it with pipetting.
- Centrifuge the tube briefly before opening the cap because the content may be on the tube wallor on the ceiling of the cap.
- Standard stock solution should be kept in ice bath during your experiment.
- Store the Standard stock solution at 0-5 °C. The solution is stable at 0-5 °C for 2 month.
Preparation of Working solution
- Add Dye Mixture to a conical tube and dilute it with Assay Buffer.
- Add Enzyme stock solution to the solution prepared in step (1).
- Refer to the Table 1.
- Working solution is light-sensitive. Prepare the solution just before use and protect it from light by covering with aluminum foil. Please use up Working solution within that da
| for 48 well | for 96 well | |
| Dye Mixture | 270 μL | 540 μL |
| Assay Buffer | 2.43 mL | 4.86 mL |
| Enzyme stock solution | 13.5 μL | 27 μL |
General Protocol
1. Sample preparation
- Prepare a cell suspension (2.5-10×105 cells) in a 1.5 mL microtube.
- Centrifuge at 300×g for 5 minutes and remove the supernatant.
- Add 500 μL of PBS to each tube, suspend by pipetting, centrifuge at 300×g for 5 minutes and remove the supernatant.
- Add 300 μL of NAD/NADH Extraction Buffer to each tube, lyse the cells by pipetting and centrifuge at 12,000×g for 5 minutes.
- Suction and discharge of the lysate using a syringe with the 25G needle (20-30 times) may work for hard-to-filter samples (e.g., viscous) to be filtered smoothly in the downstream centrifugation.
- Transfer 250 μL of the supernatant to MWCO 10K filtration tube and centrifuge at 12,000×g for 10 minutes.
- At least 100 μL of sample is required for each total NAD+/NADH and NADH measurement (Total sample requirement: 200 μL).
- Extend the centrifugation time in case the filtrate is less than 200 μL.
- Transfer 100 μL of the filtrate into two 1.5 mL microtubes and use them as total NAD+/NADH and NADH sample.
- Incubate the sample solution for measuring the amount of NADH at 60 °C for 60 minutes.
- This step is for decomposing NAD+ contained in the sample solution.
- Keep the sample solution for measuring the amount of total NAD+/NADH on ice until assaying.
- After the incubation, cool the sample solution to room temperature.
- Add 100 μL of NAD/NADH Control Buffer to each 1.5 mL microtube containing the sample solution for measuring the amount of total NAD+/NADH and NADH of Step 6. and Step 8. and use these solutions for measuring (Sample).
- 50 μL of sample per well is required.
- Please prepare samples with different dilution rate and determine the suitable dilution rate to be ranging from 0-2 μmol/L.
Use NAD/NADH Control Buffer for dilution.
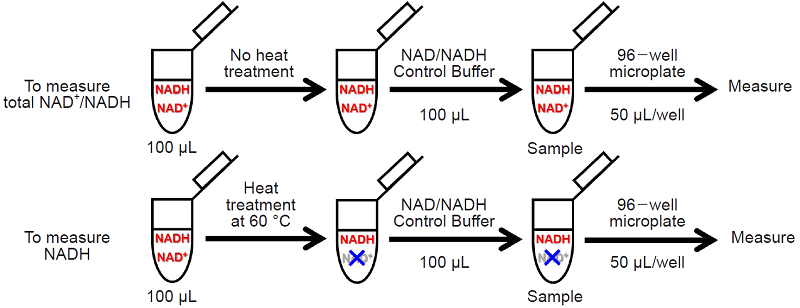
Fig. 3 Schematic diagram of the sample preparation protocol for measuring total NAD and NADH individually
2. Preparation of Standard solution
- Mix 2 μL of 10 mmol/L Standard stock solution and 198 μL of ddH2O in a microtube to prepare a 100 μmol/L Standard solution.
- Mix 10 μL of 100 μmol/L Standard solution and 490 μL of Standard Buffer in a microtube to prepare a 2 μmol/L Standard solution. Prepare Standard solutions (2, 1, 0.5, 0.25, 0.125, 0.0625, 0.0313 and 0 μmol/L) as follows by serial dilution with Standard Buffer (Fig. 4).
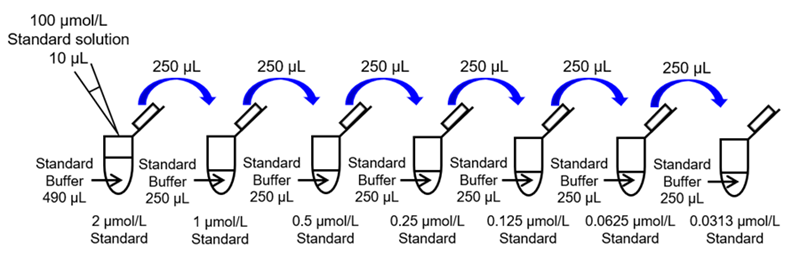
Fig. 4 Preparation of Standard solution
3. Measurement
-
- Add 50 μL of Standard solution and sample solutions to each well (Fig. 5).
- In order to obtain accurate data, we recommend triplicate measurement per sample.
- Add 50 μL of Working solution to each well.
- Since the enzymatic reaction starts immediately after the addition of Working solution to the well, use a multichannel pipette to minimize the experimental error from time lag in pipetting.
- Incubate the microplate at 37 °C for 60 minutes.
- Use a seal for the microplate to prevent evaporation of the solution during the incubation.
- Measure the absorbance at 450 nm by using a microplate reader.
- Determine the amount of total NAD+/NADH and the amount of NADH in the sample using a calibration curve.
- If the original samples were diluted for this assay, multiply the dilution rate with the determined value.
NAD+=total NAD+/NADH-NADH
- Add 50 μL of Standard solution and sample solutions to each well (Fig. 5).
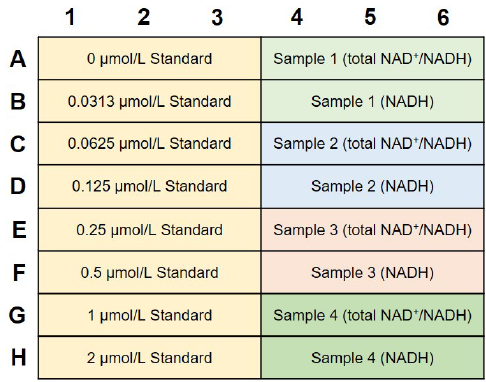
Fig. 5 An example of plate arrangement (n=3)
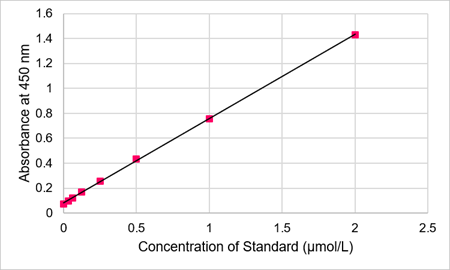
Fig. 6 Typical calibration curve of standard
Experimental Example
Analysis of total NAD+/NADH, NAD+, NADH and NAD+/NADH ratio in HeLa cells
- HeLa cell suspensions (2.5 and 5.0×105 cells/tube) were prepared in a 1.5 mL microtube.
- The supernatants were removed after centrifugation at 300×g for 5 minutes.
- PBS (500 μL) was added to each tube, and the supernatants were removed after centrifugation of the tubes at 300×g for 5 minutes.
- NAD/NADH Extraction Buffer (300 μL) was added to each tube and the cells were lysed by pipetting, and then the solutions were centrifuged at 12,000×g for 5 minutes.
- The supernatant (250 μL) was transferred to MWCO 10K filtration tube, and centrifuged at 12,000×g for 10 minutes.
- The filtrate was divided into two 1.5 mL microtubes by 100 μL, and used as the sample solution for the amount of total NAD+/NADH and NADH. The former sample solution was kept on ice until assaying.
- The sample solution for the amount of NADH was incubated at 60 °C for 60 minutes. After the incubation, the sample solution was cooled to room temperature.
- NAD/NADH Control Buffer (100 μL) was added to each 1.5 mL microtube containing the sample solution for measuring the amount of total NAD+/NADH and NADH, and these solutions were used for measuring.
- The sample solution (50 μL) and Standard solution (50 μL) were added to each well.
- Working solution (50 μL) was added to each well.
- The 96-well microplate was incubated at 37 °C for 60 minutes.
- The absorbance at 450 nm was measured by using a microplate reader, and the amount of total NAD+/NADH and NADH in the sample using a calibration curve. The amount of NAD+ was calculated by subtracting the amount of NADH from the amount of total NAD+/NADH.
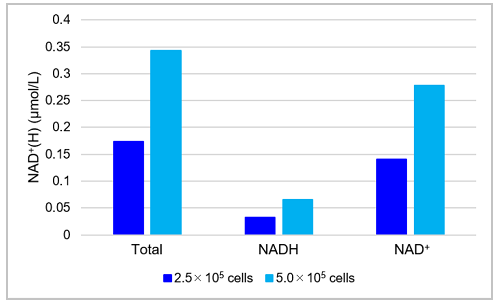
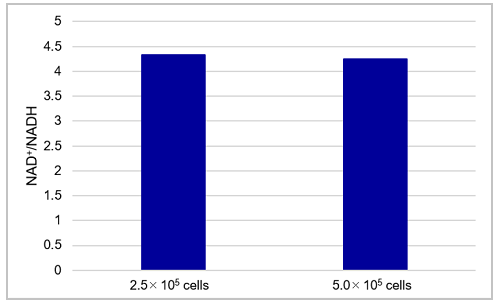
Fig. 7 The amount of total NAD+/NADH, NAD+, NADH and NAD+/NADH ratio in HeLa cells
Reference
- S.Imai, et al., Trends Cell Biol., 2014, 24, 464.
Frequently Asked Questions / Reference
N509: NAD/NADH Assay Kit-WST
Revised Oct., 27, 2023


 Hidden sections will not be printed.
Hidden sections will not be printed.