General Information
Mitochondria are major cellular organelles. They synthesize ATP via energy metabolism that depends on redoxreactions. Superoxide, a reactive oxygen species (ROS), is produced in mitochondria by electron leaking during redox reactions. Cellular control of ROS is important because ROS are associated with disease, cellular damage and senescence. The MitoBright ROS Deep Red is oxidized by mitochondrial superoxide and accumulated in mitochondria.
Content
| MitoBright ROS Deep Red | 100 nmol ×1 |
| 100 nmol ×3 |
Storage Condition
Store at 0–5°C
Required Equipment and Materials
- Fluorescence microscope, fluorescence plate reader, or flow cytometer
- Incubator (37°C)
- Microtubes
- Micropipettes (100–1000 μl, 0.5–10 μl)
- Hanks' Balanced Salt Solution (HBSS)
- Dimethyl sulfoxide (DMSO)
Precaution
- Allow the MitoBright ROS Deep Red to equilibrate to room temperature before use.
- Centrifuge the tube briefly before opening the cap because the content may be on the tube wall or the cap.
- Refer to Table 1 to check suitable fluorescence wavelengths for each application.
Table 1. Recommended filter settings for using MitoBright ROS Deep Red
| Applications | Fluorescence plate reader |
Fluorescence microscope | Flow cytometer |
| Measurement wavelength |
Ex: 535–565 nm Em: 660–690 nm |
(Confocal microscope) ・Ex/Em: 561/640–700 nm (for Single staining) ・Ex/Em: 633/640–700 nm (for co-staining with red fluorescence*) (Fluorescence microscope) ・TxRed filter |
APC filter |
- When co-stain with another red fluorescence probe, use Ex 633 nm for MitoBright ROS Deep Red to avoid signal overlap.
Preparation of Solutions
Preparation of 10 mmol/l MitoBright ROS Deep Red DMSO stock solution
Add 10 μL of DMSO to the provided tube containing MitoBright ROS Deep Red (100 nmol), and dissolve by pipetting.
- The MitoBright ROS Deep Red is difficult to see because it is present in a small amount.
Store the reconstituted MitoBright ROS Deep Red DMSO stock solution at -20°C until use. The solution is stable at -20°C for 1 month.
Preparation of MitoBright ROS Deep Red working solution
Dilute the 10 mmol/l MitoBright ROS Deep Red DMSO stock solution with culture medium or HBSS to prepare 10 μmol/l MitoBright ROS Deep Red working solution.
- The working solution cannot be stored and must be freshly prepared each day and used within the day.
Refer to Table 2 for preparation of the working solution by vessel type.
Table 2. The required amount of MitoBright ROS Deep Red working solution by vessel type
| Vessel | 35-mmdish | ibidi 8-well plate | 96-well black plate (clear bottom) |
Sample tube (Flow Cytometor) |
| Appropriate amount | 2 ml/dish | 200 μl/well | 100 μl/well | 0.5 ml/sample |
General protocol
Mitochondrial superoxide detection
- Seed cells on dishes or plates and incubate in an incubator set at 37°C equilibrated with 95% air and 5% CO2.
- Seed cells on dishes or plates and incubate in an incubator set at 37°C equilibrated with 95% air and 5% CO2.
Table 3. Recommended vessels by a detector
Detector Fluorescence plate reader Fluorescence microscope Flow cytometer Vessel 96-well black plate (clear bottom) 96-well black plate (clear bottom)
ibidi 8-well plate
35 mm dish6-well plate - Discard the culture medium and wash the cells with the culture medium.
- Add an appropriate volume of MitoBright ROS Deep Red working solution (*Table 3.) and incubate at 37°C for 30 minutes.
- Remove the supernatant and wash the cells twice with HBSS.
- Add medium or HBSS containing stimulation agents and incubate at 37°C for an appropriate time.
- Optimize the incubation time according to the stimulation conditions.
- Observe fluorescence signals.
- Do not wash the cells after step 5 as it may result in dye leakage.
Usage examples
Detection of superoxide in HeLa cells treated with Antimycin
- HeLa cells (1×105 cells/ml) in MEM (supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin) were seeded on vessels and incubated overnight in an incubator set at 37°C and equilibrated with 95% air and 5% CO2.
- The culture medium was removed and the cells were washed with HBSS twice.
- MitoBright ROS Deep Red working solution containing Antimycin (10 μmol/l) was added to the cells.
- The cells were again incubated at 37°C for 30 minutes.
- Fluorescence signals were measured by a plate reader, a flow cytometer and imagings were performed with fluorescence microscopy.
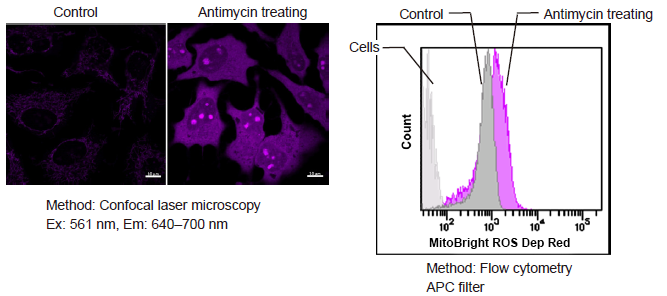
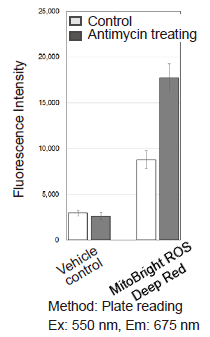
Figure 1. Fluorescence signals from HeLa cells in the presence and absence of the Complex III inhibitor Antimycin detected with a confocal laser microscope, flow cytometer and plate reader
Simultaneous analysis of mitochondrial mass, mitochondrial membrane potential and mitochondrial superoxide in HeLa cells.
- HeLa cells (3×104 cells/ml) in MEM (supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin) were seeded on ibidi-8-well plates and incubated overnight in an incubator (5% CO2, 37°C).
- The culture medium was removed and the cells were washed twice with HBSS.
- The supernatant was replaced with a working solution [Hoechst33342: 1 μg/ml, MitoTracker™ Green FM: 100 nmol/l, and Tetramethylrhodamine ethyl ester (TMRE): 150 nmol/l]and the cells were incubated at 37°C for 30 minutes.
- The supernatant was removed and the cells were washed twice with HBSS.
- The supernatant was replaced and MitoBright ROS Deep Red working solution containing Antimycin (10 μmol/l) was added to the cells.
- The cells were again incubated at 37°C for 30 minutes.
- The cells were observed under a fluorescence microscope.
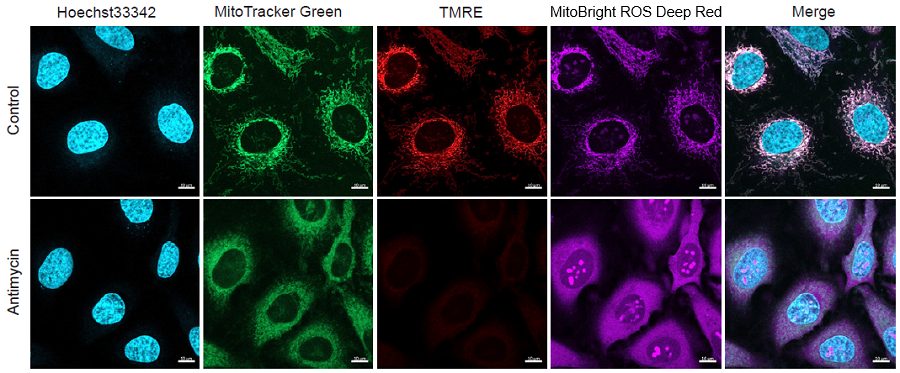
Figure 2. Simultaneous fluorescence imaging of nuclear, mitochondrial mass, mitochondrial membrane potential and mitochondrial superoxide in HeLa cells
|
Method: Confocal laser microscopy |
Supplemental Information
-
Excitation and emission spectra of MitoBright ROS Deep Red
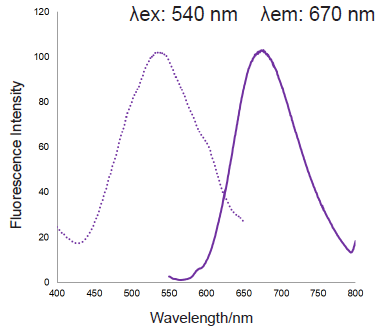
Figure 3. Excitation and emission spectra of MitoBright ROS Deep Red after reaction with superoxide
-
Selectivity of MitoBright ROS Deep Red for ROS
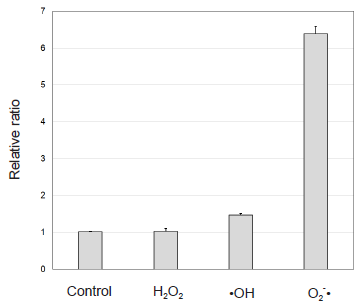
Figure 4. Selectivity of MitoBright ROS Deep Red for ROS
Comparison of detection sensitivity of MitoBright ROS Deep Red with a commonly used probe for mitochondrial superoxide indication
-
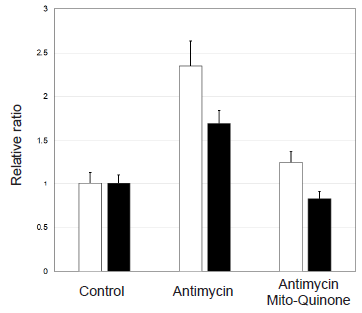
□ MitoBright ROS Deep Red ■ commonly used probe
Figure 5. Comparison of sensitivity for mitochondrial superoxide
Frequently Asked Questions / Reference
MT16: MitoBright ROS Deep Red - Mitochondrial Superoxide Detection
Revised Feb., 27, 2024


 Hidden sections will not be printed.
Hidden sections will not be printed.