General Information
Mitochondria are an important organelle that uses oxygen to synthesize ATP, producing the necessary energy for living cells to thrive1). Decreased mitochondrial activity and mitochondrial dysfunction are associated with cancer, aging, and neurodegenerative diseases such as Alzheimer’s and Parkinson’s disease2,3). Therefore, mitochondrial membrane potential (MMP) has been widely studied as a promising target for mitochondria-related diseases.
JC-1 dye, tetramethylrhodamine ethyl ester (TMRE), and tetramethylrhodamine methyl ester (TMRM) are widely used to monitor MMP, however, these dyes have some limitations, such as low photostability and poor retention after aldehyde fixation. These limitations result in poor reproducibility of experiments.
Dojindo’s MT-1 MitoMP Detection Kit overcomes these limitations. This kit can monitor MMP with excellent reproducibility even after aldehyde fixation. Furthermore, the MT-1 Dye in this kit is extremely photostable and more sensitive than JC-1. In addition, the Imaging Buffer included in this kit minimizes background fluorescence and maintains cell vitality while the assay is being performed.
Contents
| MT-1 Dye | 20 μl x 3 |
| Imaging Buffer (10x) | 11 ml x 1 |
Storage Condition
Store at -20℃, protected from light.
Required Equipment and Materials
- Growth medium or HBSS
- Micropipettes (100–1000 μl, 0.5–10 μl)
- Microtubes
Preparation of Solutions
Preparation of Imaging Buffer solution
Dilute the 10x Imaging Buffer (1:10) in double-deionized water.
- Use the Imaging Buffer solution within the same day.
Table 1 Required amount of Imaging Buffer solution by vessel type
| Vessel | 35-mm dish | ibidi 8-well plate |
| Appropiate amount | 2 ml/dish | 200 μl/well |
| Vessel | 96-well plate | Sample tube (Flow Cytometry) |
| Appropiate amount | 100 μl/well | 0.5 ml/sample |
Preparation of MT-1 working solution
Dilute the MT-1 Dye (1:1000) in the cell culture medium.
- Use the MT-1 working solution within the same day.
General Protocol
- Inoculate cells into a dish or chamber slide and incubate in an incubator set at 37 ℃ and equilibrated with 95% air and 5% CO2.
- Add an appropriate volume of MT-1 working solution to cells.
- Incubate the cells for 30 minutes in an incubator set at 37 ℃ and equilibrated with 95% air and 5% CO2.
- Discard the supernatant and wash the cells with medium or HBSS twice.
- Induce a change in MMP.
- Discard the supernatant and wash the cells with medium or HBSS twice.
- Add Imaging Buffer solution and observe the cells under a fluorescence microscope.
Usage Examples
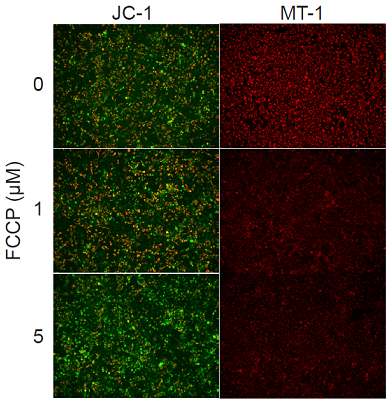
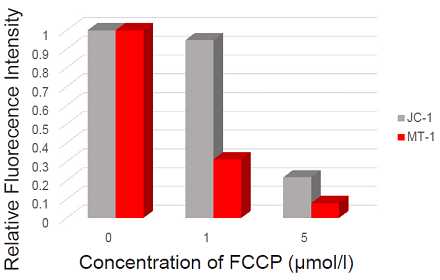
Fluorescence microscopic detection of MMP in HeLa cells treated with carbonyl cyanide-p-trifluoromethoxyphenyl hydrazone (FCCP)
- HeLa cells (2.4x105 cells/ml, 100 μl) in MEM (supplemented with 10% fetal bovine serum, and 1% penicillin-streptomycin) were seeded in a 96-well clear-bottom black plate and cultured overnight in an incubator set at 37 ℃ and equilibrated with 95% air and 5% CO2.
- After the supernatant (100 μl) was removed, the MT-1 working solution (1:1000 MT-1 diluted in MEM, 100 μl) was added. The cells were cultured for 30 minutes in an incubator set at 37 ℃ and equilibrated with 95% air and 5% CO2.
- The cells were washed with 100 μl of HBSS twice.
- FCCP in MEM (0, 1, or 5 μmol/l, 100 μl) was added to each well, and then the cells were cultured for 30 minutes in an incubator set at 37 ℃ and equilibrated with 95% air and 5% CO2.
- The cells were washed with 100 μl of HBSS twice.
- Imaging Buffer solution (100 μl) was added, and the cells were observed under a fluorescence microscope.
- The imaging data were quantified using Macro Cell Count (KEYENCE).
MT-1: 530-560 nm (Ex), 570-640 nm (Em)
JC-1:
Green 450-490 nm (Ex), 500-550 nm (Em)
Red 530-560 nm (Ex), 570-640 nm (Em)
Figure 1. Fluorescence imaging of mitochondrial membrane potential in HeLa cells.
Usage Examples
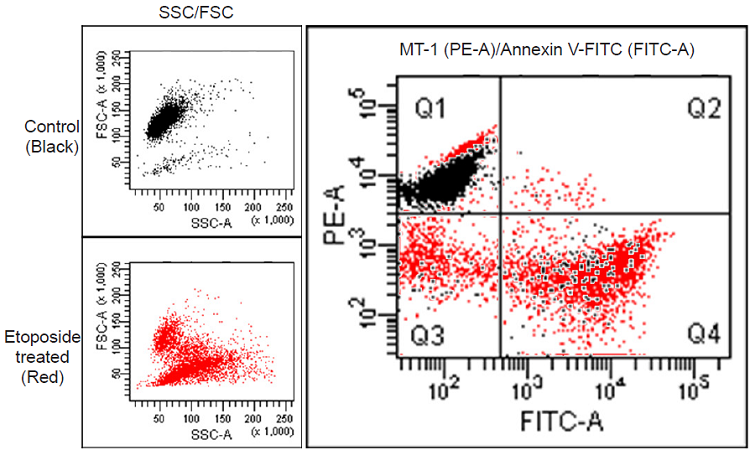
Flow cytometry analysis of mitochondria in apoptosis-induced HL60 cells.
- HL60 cells (1.0x106 cells/ml, 1 ml) in RPMI (10% fetal bovine serum, 1% penicillin-streptomycin) were transferred to a 1.5 ml tube.
- The cells suspension was centrifuged at 200xg for 3 minutes, and then the medium was removed and the MT-1 working solution (1:1000 MT-1 diluted in RPMI, 1 ml) was added. Next, the cells were incubated for 30 minutes in an incubator set at 37 ℃ and equilibrated with 95% air and 5% CO2.
- The cells suspension was centrifuged at 200xg for 3 minutes, and then the cells were washed with 100 μl of HBSS twice.
- Etoposide in RPMI (0 or 50 μmol/l, 1 ml) was added, and cells were incubated for 24 hours in an incubator set at 37 ℃ and equilibrated with 95% air and 5% CO2.
- The sample (100 μl) was transferred to another 1.5 ml tube and 5 μl of FITC Annexin V (Becton Dickinson, Cat. No. 51-65874X) was added, followed by incubation for 15 minutes in an incubator set at 37 ℃ and equilibrated with 95% air and 5% CO2.
- Imaging Buffer solution (400 μl) was added, and the cells were analyzed using a flow cytometer.
| <Detection> Annexin V-FITC: 488 nm (Ex), 515–545 nm (Em) MT-1: 488 nm (Ex), 564–604 nm (Em) |
Figure 2. Flow cytometric analysis of MMP in HL60 cells.
References
- Ferri, K. F. et al., J. Exp. Med., 2000, 192, 1081–1092.
- Matsuda, N. et al., J. Cell Biol., 2010, 189, 211.
- Wang, J. L. et al., PNAS, 2000, 97, 7124–7129.
Frequently Asked Questions / Reference
MT13: MT-1 MitoMP Detection Kit
Revised May., 23, 2023


 Hidden sections will not be printed.
Hidden sections will not be printed.