General Information
MitoPeDPP is a newly developed fluorescent dye which can penetrate cell membranes and is accumulated in the mitochondria due to the triphenylphosphonium moiety. MitoPeDPP accumulated in mitochondrial inner membranes is oxidized by lipophilic peroxide and then emits strong fluorescence. Since the excitation and emission wavelengths of the oxidized MitoPeDPP (Ox-MitoPeDPP) are 452 nm and 470 nm, respectively, photodamage and autofluorescence of the samples can be minimized. Therefore, MitoPeDPP can be applicable for imaging of the lipophilic peroxide in living cells under a fluorescence microscope.
*This product was developed by Dr. Shioji, Department of Chemistry, Fukuoka University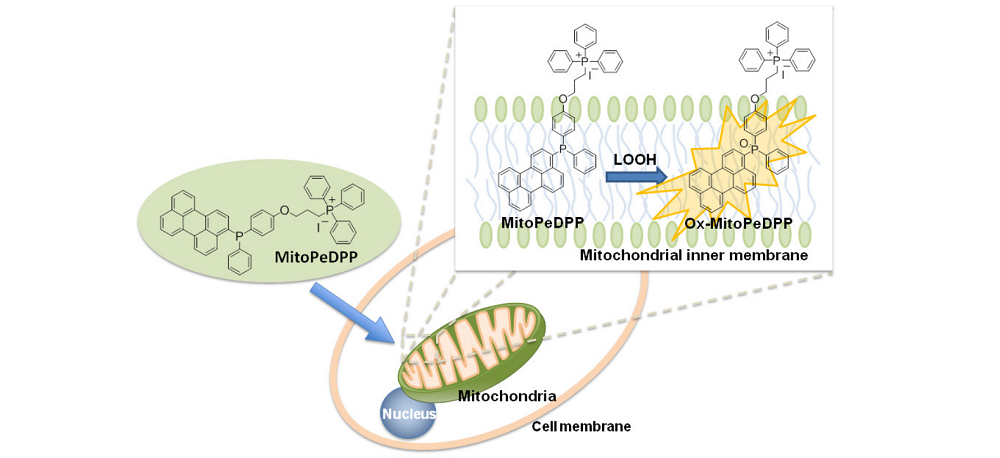
Contents
MitoPeDPP 5 μg x 3
- The pellet in the tube may be barely visible due to the small amount of MitoPeDPP. Check the color of the MitoPeDPP DMSO solution is yellow.
Storage Condition
Store at 0-5oC and protect from light.
- Close the bag tightly and store at 0-5oC after opening the bag.
Required Equipment and Materials
- Dimethyl sulfoxide (DMSO)
- PBS
- Hanks’ HEPES buffer
- Micropipettes
Preparation of Solutions
Preparation of 0.1 mmol/l MitoPeDPP DMSO solution
Add 50 μl DMSO to a tube and dissolve 5 μg MitoPeDPP with pipetting.
- Since MitoPeDPP is not stable in DMSO, protect the solution from light and use it within the same day.
Preparation of MitoPeDPP working solution
Dilute the MitoPeDPP DMSO solution with Hanks’ HEPES buffer to prepare 0.1-0.5 μmol/l MitoPeDPP working solution.
- Hanks’ HEPES buffer is recommended to maintain cell condition stable.
- Prepare the working solution immediately prior to adding it to cells to avoid the autooxidation of MitoPeDPP.
General Protocol
MitoPeDPP Staining
- Prepare cells for the assay.
- Discard the culture medium and wash the cells with either Hanks’ HEPES buffer or PBS twice.
- Add an appropriate volume of MitoPeDPP working solution.
Type of dishes Volume of working sol. 35-mm dish 2000 μl 96 well plate 100 μ - Incubate at 37 oC for 15 minutes with protection from light.
- Discard the supernatant and wash the cells with either Hanks’ HEPES buffer or PBS twice.
- Add Hanks’ HEPES buffer or PBS containing a stimulating agent and observe the cells under a fluorescence microscope.
- Filter (wavelength/band pass): 470/40 (Ex), 525/50 (Em)
- Since the time of generating lipophilic peroxide in cells vary depending on the type of cells or stimulating agents, please observe time-dependent change under a fluorescence microscope after adding the agent.
Notes
This protocol is given as a general method. Please optimize staining conditions such as a suitable final concentration of MitoPeDPP and exposure time of stimulating agents.
Supplemental Information
Detection of lipophilic peroxide genetated with rotenone
HeLa cells were seeded on a μ-slide 8 well (Ibidi) and cultured at 37 oC in a 5%-CO2 incubator overnight. After the cells were washed with 200 μl Hanks’ HEPES buffer twice, 0.1 μmol/l MitoPeDPP working solution was added to the μ-slide. The cells were then incubated at 37 oC for 15 minutes. After the washing of cells with Hanks’ HEPES buffer twice, the μ-slide filled with 200 μl of Hanks’ HEPES buffer was set under a fluorescent microscope. Two hundred μl (200 μl) of 2 μmol/l rotenone in Hanks’ HEPES buffer was added to the cells (final concentration of rotenone: 1 μmol/l) and time dependent fluorescence change was observed under a fluorescence microscope for 3 hours.
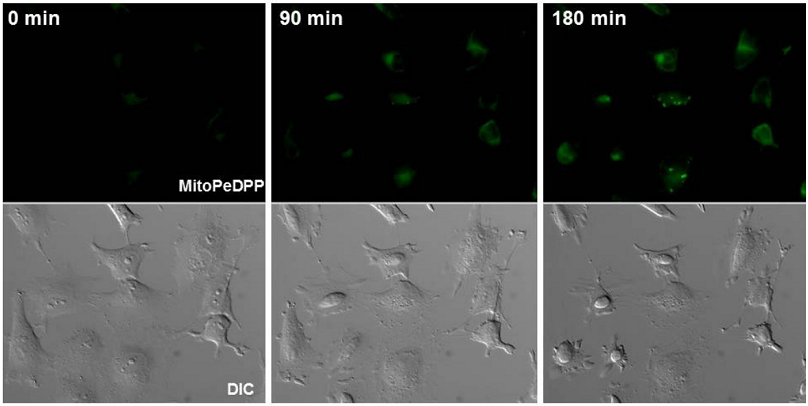
Figure 1 Fluorescent images of HeLa cells after stimulation with rotenone
Exposure time of rotenone: 0 minute (left), 90 minutes (center), 180 minutes (right)
Selectivity of MitoPeDPP reaction to various ROS (reactive oxygen species) and RNS (reactive nitrogen species)
In a homogeneous system, MitoPeDPP reacts with various peroxides such as H2O2 , t-BHP, and ONOO-. On the other hands, Fig. 2A and 2B show that MitoPeDPP accumulated in mitochondria is oxidized by t-BHP but not with other ROS or RNS.
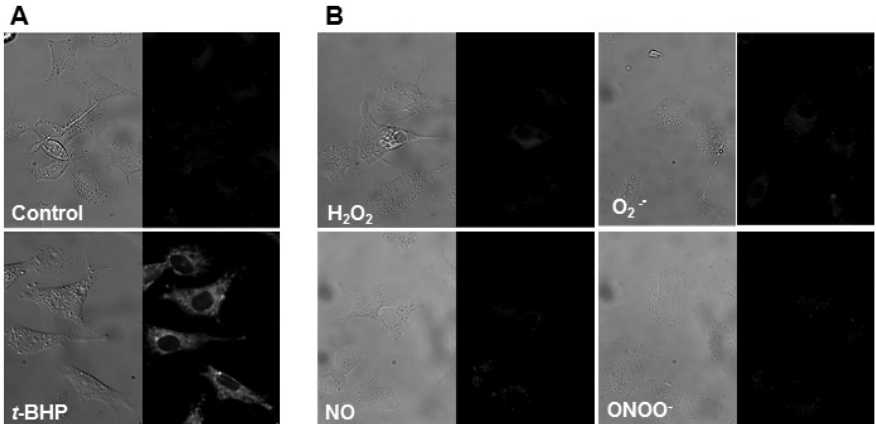
Figure 2
A) Cells were incubated with MitoPeDPP for 15 minutes and then treated with 100 μmol/l t-BHP. The fluorescent image was taken after 15 minutes incubation. These photos shows the phase contrast (left) and fluorescence (right) images of cells with or without t-BHP.
B)Cells exposed to MitoPeDPP were treated with the ROS and RNS generating reagents. The concentrations of each ROS and RNS were 100 μmol/l (H2O2 , NO, and ONOO-) and 10 μmol/l (O2 ࣭- ). PMA was used as a O2 ࣭- generator.
- t-BHP, tert-Butylhydroperoxide; PMA, Phorbol myristate acetate; SIN-1, 3-(Morpholinyl)sydnonimine, hydrochloride; NOC 7, 1-Hydroxy-2-oxo-3-(N-methyl-3-aminopropyl)-3-methyl-1-triazene
- Filter (wavelength/band pass): 470/40 (Ex), 525/50 (Em)
Reference
- K. Shioji et al., ‘’Synthesis and properties of fluorescence probe for detection of peroxides in mitochondria’’, Bioorg. Med. Chem. Lett., 2010, 20, 3911.
Frequently Asked Questions / Reference
M466: MitoPeDPP
Revised Aug., 22, 2023


 Hidden sections will not be printed.
Hidden sections will not be printed.