General Information
Liquid-liquid phase separation (LLPS) is a phenomenon where two liquids with different compositions do not mix and instead separate into two distinct phases. Although this phenomenon has been well-known in physical chemistry for a long time, it has only recently been observed in living organisms, bringing an important new perspective to various biological processes. LLPS is involved in almost all intracellular phenomena and is actively studied in neurodegenerative diseases. For example, in Parkinson’s disease, frontotemporal lobar degeneration, Alzheimer’s disease, and amyotrophic lateral sclerosis—diseases caused by intracellular protein aggregation (misfolding diseases)— the discovery of LLPS involving droplet formation of α-synuclein, FUS, tau, and TDP-43 has provided new clues to help elucidate biological phenomena.1) Clarifying the involvement of LLPS in neurodegenerative diseases is expected to lead to a better understanding of their pathogenesis and the development of novel therapeutic strategies.
This kit includes four dyes—two amyloid staining dyes (Thioflavin T, Congo Red) and two that respond to hydrophobic interactions between proteins (ANS, SepaFluor). Additionally, using SepaFluor, we can observe the gelation of phase-separated droplets. Using these dyes leads to a better understanding of the properties of phase-separated droplets.
- This kit is not applicable to phase-separated droplets in cells; please use this kit for cell-free experiments.
- This kit does not include standard proteins, crowding agents, buffers, etc.
| Contents | Properties |
| ANS (8-anilino-1-naphthalenesulfonic acid) | Dye that emits blue fluorescence in a hydrophobic field |
| SepaFluor |
Dye that emits purple fluorescence in a hydrophobic field. |
| Thioflavin T | Dye that binds to amyloid and emits green fluorescence |
| Congo Red | Dye that binds to amyloid and emits red fluorescence |
Kit Contents
|
ANS |
50 µl x 1 |
| SepaFluor | 10 nmol x 1 |
| Thioflavin T (10 mmol/l DMSO solution) |
50 µl x 1 |
| Congo Red (10 mmol/l DMSO solution) |
50 µl x 1 |
Storage Conditions
Store at -20°C and protected from light.
Required Equipment and Materials
- Target protein, etc.
- Crowding agents, buffer, etc.
- Dimethylsulfoxide (DMSO)
- Electronic balance
- Micropipette
- Microtube
- Conical tube
- Vortex mixer
- Fluorescence microscope
- Slide glass
- Cover glass
- Double-Sided Tape
- 96-well black microplate (clear bottom)
- Please refer to Q&A for available plates.
Preparation of Solutions
0.1 mmol/l Preparation of SepaFluo DMSO solution
Add 100 μl of DMSO to a tube of SepaFluo (10 nmol) and dissolve by vortexing.
- Store the DMSO stock solution at -20 °C. The DMSO stock solution is stable at -20 °C for 6 months.
- ANS, Thioflavin T, and Congo Red are provided as solutions in DMSO and do not require this procedure.
General Protocol
Add the DMSO solution of the dye to the phase separation solution so that the final concentration is a 1000-fold dilution.
There are several methods for making and observing phase-separated droplets. Here, we introduce methods using 96-well plates and slide glasses.
Observation Using 96-well Microplate
For observation with 96-well plates, detailed procedures can be found in Experimental Example 1, 2.
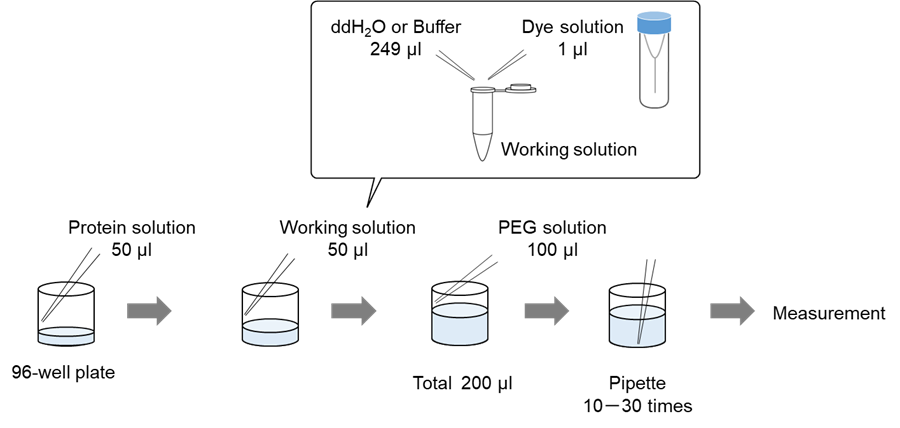
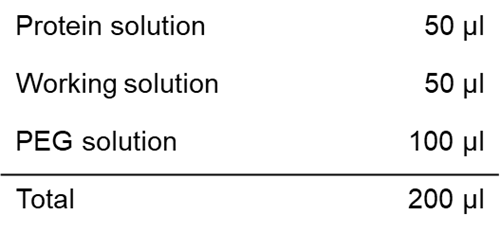
Solution preparation example
Observation Using slide glasses
For observation with slide glasses, detailed procedures can be found in Experimental Example 3.
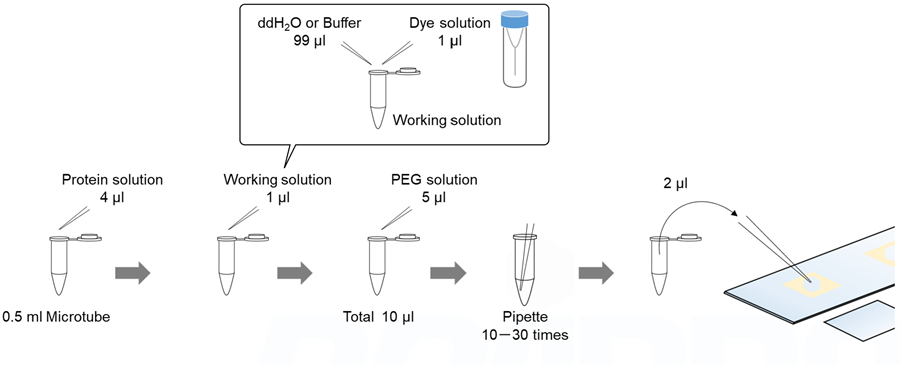
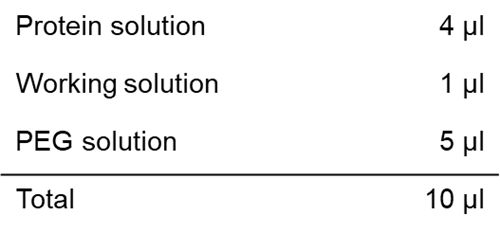
Solution preparation example
Usage notes
- The experiments described in this instruction manual were performed with 1 hour of staining. Although the staining time varies depending on the type of phase-separated droplets, the staining time should be standardized when comparing conditions. For staining over a longer period of time, please refer to the FAQ.
- Use double-deionized H2O (ddH2O) or buffer to prepare the working solution. In the experiments described in this instruction manual, the DMSO solution of the dye was diluted with ddH2O.
- The number of pipetting actions can affect phase-separated droplet formation. Ensure consistency in the number of pipetting actions for each procedure.
- The number of phase-separated droplets may increase or decrease depending on the temperature. Maintain a consistent room temperature for each experiment.
- Depending on the sample type, droplets may spread and wet the bottom of the slide glass or plate, making it difficult to observe spherical droplets. If wetting occurs, consider applying a coating to enable observation of spherical droplets. Alternatively, observe droplets that are not in contact with the bottom surface.
Experimental Example 1
Staining of droplets of BSA (bovine serum albumin) prepared using the crowding agent PEG 8000
Droplets of BSA prepared with the crowding agent PEG 8000 were stained with ANS and SepaFluor.
Preparation of a working solution
A working solution was prepared by diluting 1 µl of ANS and SepaFluor DMSO solution with 99 µl of ddH2O.
Preparation of BSA solution
A 1000 μmol/l aqueous solution of BSA (A2153-10MG, Sigma–Aldrich) was prepared with ddH2O.
Preparation of PEG8000 solution
A 30 w/v% PEG 8000 solution was prepared with ddH2O.
- PEG8000 was used in the (LL02) LLPS Forming Condition Screening Kit.
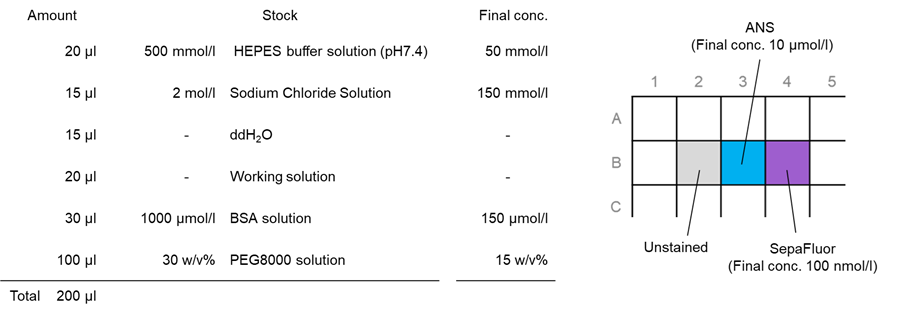
Preparation, staining, and observation of phase-separated solutions of BSA
1. Twenty (20) µl of 500 mmol/l HEPES Buffer Solution (pH 7.4) was added to each well in a 96-well black microplate (clear bottom, 5882-096: AGC Techno Glass).
- 500 mmol/l HEPES Buffer Solution was used in the (LL02) LLPS Forming Condition Screening Kit.
2. Fifteen (15) µl of 2 mol/l Sodium Chloride Solution was added to each well.
- 2 mol/l Sodium Chloride Solution was used in the (LL02) LLPS Forming Condition Screening Kit.
3. Fifteen (15) µl of ddH2O was added to each well.
4. Twenty (20) µl of ANS and SepaFluor working solution was added to each well.
5. Thirty (30) μl of 1000 μmol/l BSA solution was added to each well.
6. One hundred (100) µl of 30 % PEG 8000 solution was added to each well; 20 pipetting cycles were performed.
- Because of its high viscosity, the PEG8000 solution should be added slowly using reverse pipetting.
- Manual pipetting is strongly recommended to avoid the foaming that tends to occur when using electric pipettes.
- After adding the PEG solution, promptly pipette to prevent protein aggregation if left standing for an extended period.
7. The samples were incubated at room temperature for 1 hour and protected from light; after incubation, the solution was observed under an epi-illumination fluorescence microscope.
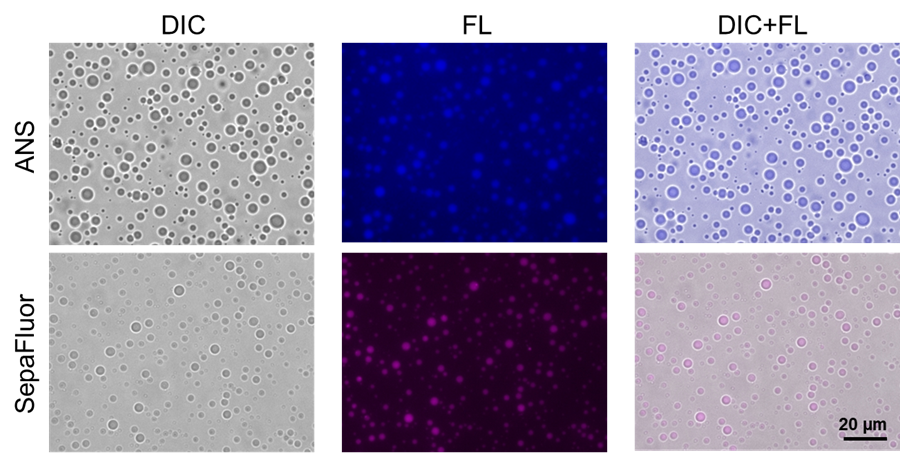 |
| Fig. 1 Staining of droplets of BSA prepared using the crowding agent PEG 8000 |
Filter sets
ANS: 340–380 nm (Ex), 435–485 nm (Em)
SepaFluor: 590–650 nm (Ex), 663–737 nm (Em)
Experimental Example 2
Staining of phase-separated droplets of lactoferrin prepared using the crowding agent PEG8000
Experiments were performed with lactoferrin protein, which is known to form phase-separated droplets mainly by electrostatic interaction. As the concentration of PEG8000 is increased, the droplets of lactoferrin become gelatinized. The changes were observed with SepaFluor.
Preparation of a working solution
A working solution was prepared by diluting 1 µl of SepaFluor DMSO solution with 249 µl of ddH2O.
Preparation of lactoferrin solution
Eighty (80) μmol/l aqueous solution of lactoferrin (L4040-100MG, Sigma–Aldrich) was prepared with 80 mM HEPES (pH 7.4).
Preparation of PEG8000 solution
A 10 and 20 w/v% PEG8000 solution were prepared with ddH2O.
- PEG8000 was used in the (LL02) LLPS Forming Condition Screening Kit.
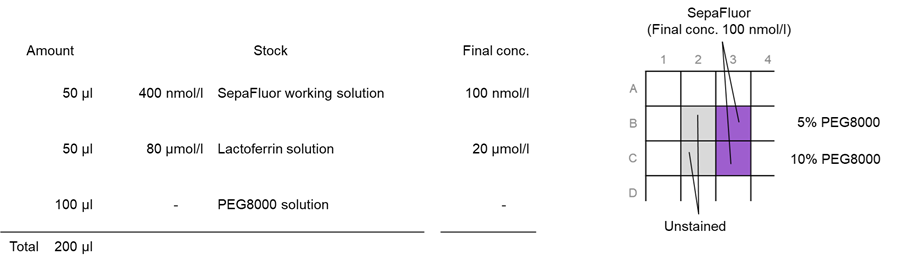
Preparation, staining, and observation of phase-separated solutions of lactoferrin
1. Fifty (50) µl of SepaFluor working solution was added to each well in a 96-well black microplate (clear bottom, 5882-096: AGC Techno Glass).
2. Fifty (50) µl of 80 μmol/l lactoferrin solution was added to each well.
3. One hundred (100) µl of 10 % and 20 % PEG8000 solution was added to each well; 20 pipetting cycles were performed.
- Because of its high viscosity, the PEG8000 solution should be added slowly using reverse pipetting.
- Manual pipetting is strongly recommended to avoid the foaming that tends to occur when using electric pipettes.
- After adding the PEG solution, promptly pipette to prevent protein aggregation if left standing for an extended period.
4. The samples were incubated at room temperature for 1 hour and protected from light; after incubation, the solution was observed under an epi-illumination fluorescence microscope.
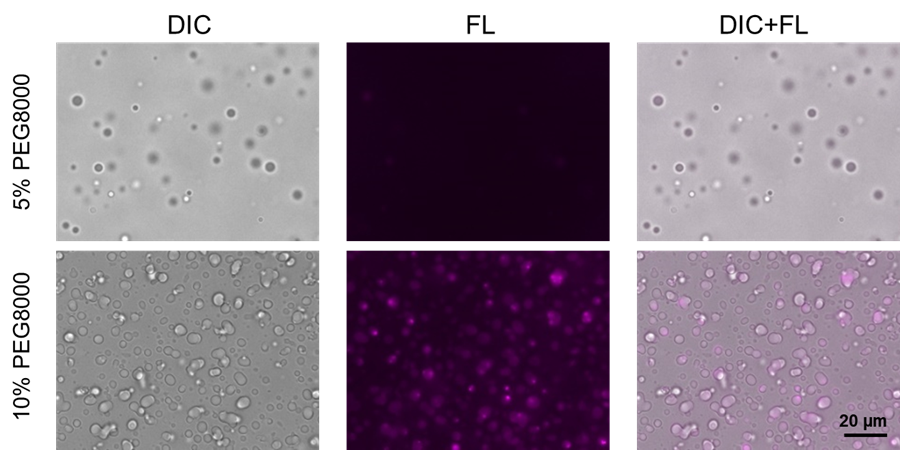 |
| Fig. 2 Staining of phase-separated droplets of lactoferrin prepared using the crowding agent PEG8000 |
Filter sets:590–650 nm (Ex), 663–737 nm (Em)
Experimental Example 3
Staining of Tau aggregates with Heparin
Tau protein aggregates prepared with Tau protein and Heparin were stained with Thioflavin T and Congo Red.
Preparation of a working solution
A working solution was prepared by diluting 1 µl of Thioflavin T and Congo Red DMSO solution with 99 µl of ddH2O.
Preparation of Heparin solution
A 40 % (w/v) aqueous solution of Heparin (081-00136, FUJIFILM Wako Pure Chemical) was prepared with ddH2O.
Preparation of Buffer solution
Mixed the 1.5 µl of 500 mmol/l HEPES Buffer Solution (pH 7.4), 6.4 µl of 2 mol/l Sodium Chloride Solution, and 9.2 µl of ddH2O in a 0.5 ml microtube.
- 500 mmol/l HEPES Buffer Solution and 2 mol/l Sodium Chloride Solution were used in the (LL02) LLPS Forming Condition Screening Kit.
Preparation, staining, and observation of Tau aggregates
1. One point seven one (1.71) µl of Buffer solution was added to each 0.5 ml microtube.
2. One (1) µl of Thioflavin T and Congo Red working solution was added.
3. Two point two nine (2.29) µl of Tau solution (SPR-479B, StressMarq Biosciences) was added to each 0.5 ml microtube.
4. Five (5) µl of 40 % Heparin solution was added to each 0.5 ml microtube; 20 pipetting cycles were performed for each 0.5 ml microtube.
- Because of its high viscosity, the Heparin solution should be added slowly using reverse pipetting.
- Manual pipetting is strongly recommended to avoid the foaming that tends to occur when using electric pipettes.
- After adding the Heparin solution, promptly pipette to prevent protein aggregation if left standing for an extended period.
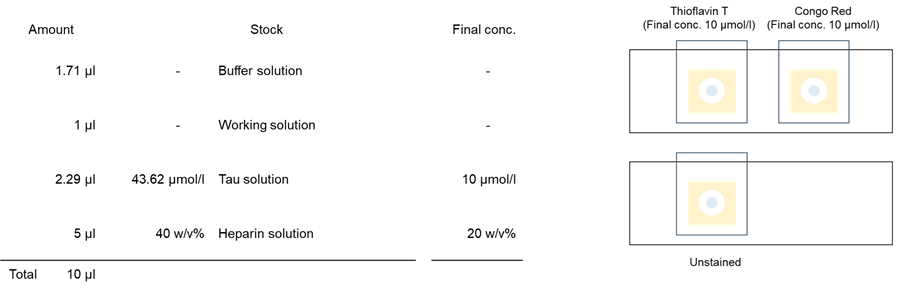
5. Two (2) µl of sample solution was added to a glass slide with double-sided tape with a hole. The glass slides were covered with a cover glass and incubated at 37°C for 7 days, protected from light. After incubation, the samples were observed under a confocal fluorescence microscope.
- The same glass slides, cover glasses, and double-sided tape were used as in the (LL01) LLPS Starter Kit.
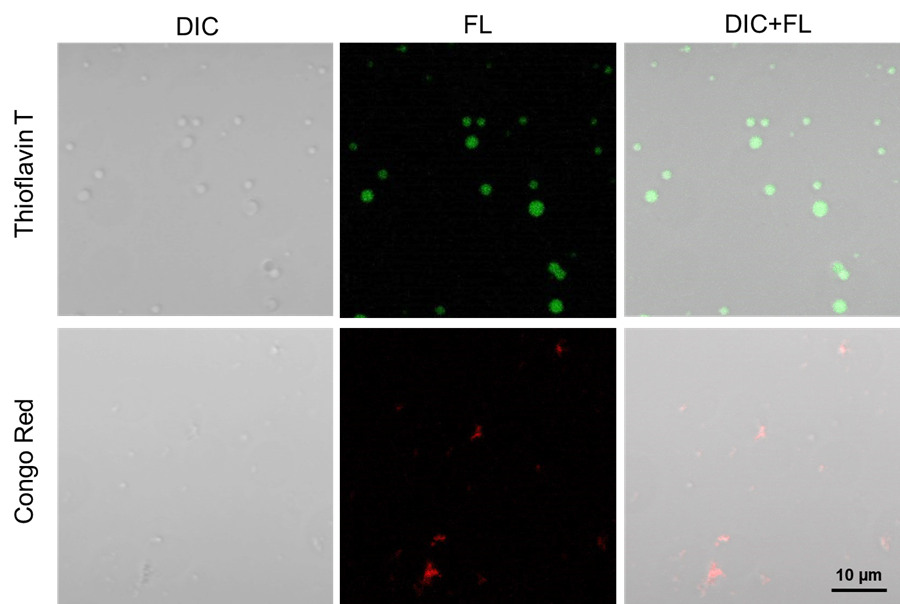 |
| Fig. 3 Staining of Tau aggregates with Heparin |
Filter sets
Thioflavin T: 488 nm (Ex), 500–600 nm (Em)
Congo Red: 561 nm (Ex), 570–700 nm (Em)
References
- A. Zbinden et al., Dev Cell., 2020, 55, 45-68.
Frequently Asked Questions / Reference
LL03: LLPS Characterization-dye Set
Revised Nov., 11, 2024


 Hidden sections will not be printed.
Hidden sections will not be printed.

