General Information
R-Phycoerythrin Labeling Kit-SH is primarily used for the preparation of R-Phycoerythrin-labeled antibody for immunostaining
and cellular proteins for tracing.SH-Reactive R-Phycoerythrin, a component of this kit, has maleimide groups that react with sulfhydryl groups (SH) of reduced IgG or other proteins.This kit contains all of the necessary reagents for the labeling. Reducing Agent in this kit produces free sulfhydryl groups in the IgG molecule without loss of antibody affinity.The labeling process is simple. After the reducing reaction, add SH-Reactive R-Phycoerythrin to IgG solution on the membrane of Filtration Tube, and incubate at 37℃for 1h.The maximum excitation and emission wavelengths of the R-phycoerythrin-labeled proteins are 564 nm and 575 nm, respectively.This kit contains all of the necessary reagents for labeling, including the storage buffer for conjugates.
Kit Contents
-
SH-Reactive R-Phycoerythrin 3 tubes RA Solution 1 ml x 1 WS Buffer 4 ml x 1
-
Reducing Agent 3 tubes Reaction Buffer 200 μL x 1 Filtraion Tube 3 tubes
Capacity
Three samples labeling
- Sample requirement: Molecular weight > 50,000; amount: 50-200 μg
Storage Condition
Store at 0-5°C. This kit is stable for 1 year at 0-5°C before opening.
|
Caution After a SH-Reactive R-Phycoerythrin is taken out from the seal bag,keep the unused SH-Reactive R-phycoerythrin(s) in the bag, sealtightly and store at -20 °C. Store the other components at 0-5°C. |
Required Equipment
- 200 μl adjustable pipettes
- Incubator (37°C)
- Microcentrifuge
- Microtubes
Precaution
- If the target protein has free sulfhydryl groups, skip the reducing procedure (Step 3-6).
- If the target protein solution contains other proteins with molecular weight larger than 10,000, such as serum albumin or gelatin, purify the protein solution, and use the purified target proteins for R-phycoerythrin labeling, because it might interfere the filtering or labeling reaction.
- If the protein solution contains small insoluble materials, centrifuge the solution, and use the supernatant for the labeling.
- The amount of Reducing Agent is optimized for the preparation of the reduced IgG. Please examine the necessary amount of Reducing Agent for the reduction of other proteins.
- The droplets which induced from the dry inhibitor of membrane, are occasionally found inside Filtration Tube while storing the kit at 0-5 ℃ or after returning to room temperature. This phenomenon does not affect the performance.
- This kit includes microtubes containing solutions. Since there is a possibility that the droplets might attach to the inside walls or caps, please shake them down prior to open.
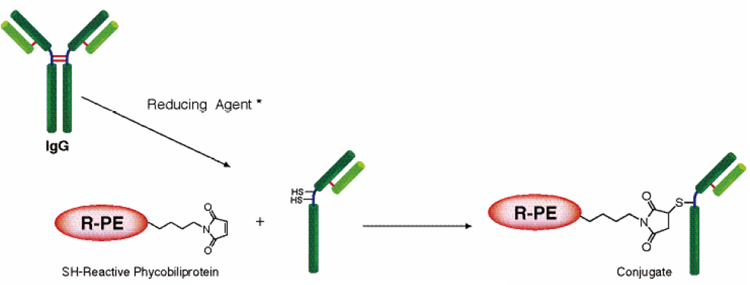
Disulfides that are not in the hinge region may be reduced.
|
|
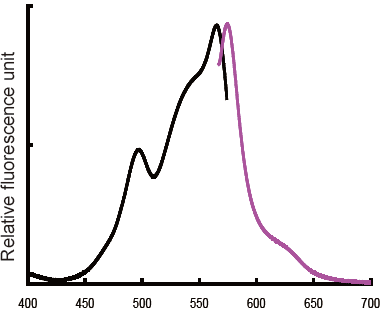
Maximum excitation wavelength : 564 nm |
Excitation and emission spectra of R-Phycoerythrin-labeled protein
General Protocol
Labeling for IgG
-
Step 1.
Add 100 μL WS Buffer and the sample solution containing 50-200 μg IgGa) to a Filtration Tube.
Step 2.
Pipette to mix and centrifuge at 8,000 x g for 10 min.b)
-
Step 3.
Add 150 μL WS Buffer to a tube of Reducing Agent, and dissolve with pipetting.
Step 4.
Transfer 100 μL of the Reducing Agent solution onto the membrane of Filtration Tube, and pipette to dissolve the IgG.
-

Step 5.
Incubate the tube at 37°C for 30 min. Add 100 μL RA Solution, and centrifuge at 8,000 x g for 10 min.b)
Step 6.
After discard the filtrate, add 200 μL RA Solution, and centrifuge at 8,000 x g for 10 min again.b)
-
Step 7.
Add 50 μL Reaction Buffer to SHReactive R-Phycoerythrin, and dissolve it with pipetting.c)
Step 8.
Transfer SH-Reactive R-Phycoerythrin solution to the Filtration Tube,and pipette to mix.
-

Step 9.
Incubate the tube at 37°C for 1 h.
Step 10.
Add 150 μL WS Buffer, and pipette about 10 times to recover the conjugate.e) Transfer the solution to a microtube (not included in this kit), and store at 0-5°C.
a) The volume of IgG solution should be less than 100 μL. If the IgG concentration is lower than 0.5 mg/mL, repeat Steps 1 and 2 until the total IgG accumulation becomes 50-200 μg.
b) If solution still remains on the membrane after the centrifugation, spin for another 5 min.
c) SH-Reactive R-Phycoerythrin is unstable in Reaction Buffer. Proceed to Step 8 immediately after the preparation of the SH-Reactive R-Phycoerythrin solution.
d) One to two R-phycoerythrin should be introduced into one reduced IgG molecule. Unconjugated R-phycoerythrin remained in the solution might cause background increase with immunoassay. If purification is necessary, purify the conjugate using a gel permeation column or an affinity column for IgG.
e) We recommend using WS Buffer to storage the conjugate. You can choose any kinds of buffers appropriate for your experiment.
Q&A
Can I use this kit to label antibody which is commercially available?
Yes. However, if antibody solution contains other proteins such as serum albumin or gelatin, labeling reaction might be interfered by that
protein. Purification of the antibody solution with affinity chromatography is necessary prior to use this kit. Contact us for the purification
procedure, if you need.
What is the minimum amount of protein that can be labeled using this kit?
We recommend using 50 μg as a minimum amount. Though 10 μg protein can be labeled using this kit, the background might be
increased.
Can I use the R-phycoerythrin conjugated protein that is precipitated in storage?
Yes. The precipitated protein should be removed by centrifugation at 10,000 x g for 10 min, and use the supernatant.
Is there any notice for treatment of living cells with the R-phycoerythrin conjugated protein?
We recommend using PBS including 2-10% FBS for preparation of cell suspension to maintain the best cell condition.
Does recovery buffer (WS Buffer) have harmful effect to living cells?
No. WS Buffer contains stabilizing agent (surfactant) that is controlled of its concentration without cytotoxicity.
If you are concerned about the additive in WS Buffer, you can use your own buffer currently used instead of WS Buffer.
Frequently Asked Questions / Reference
LK26: R-Phycoerythrin Labeling Kit - SH
Revised Nov., 24, 2023


 Hidden sections will not be printed.
Hidden sections will not be printed.