General Information
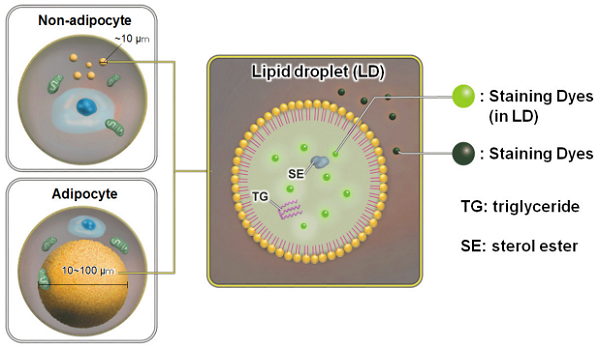
Lipid droplets (LDs) are composed of neutral lipids such as triacylglycerol and cholesteryl esters surrounded by a phospholipid monolayer, and are found not only in adipocytes but also ubiquitously in eukaryotic organisms.1) Although LDs were originally considered to be a type of lipid storage machinery, a recent study has shown that LDs play an important role in regulating lipid metabolism, autophagy2), cellular senescence3), and differentiation.4)
Oil Red O and Nile Red staining are widely used to detect LDs. The method by using Oil Red O has the following disadvantages: 1) time-consuming and complicated procedure, 2) Oil Red O tends to precipitate in solution, 3) precipitated Oil Red O may cause errors of measurement, and 4) only applicable for fixed cells. On the other hand, the method by using Nile Red partelly overcome the disadvantages of Oil Red O. However, Nile Red is not well-suite method for lipid droplet assay due to its high background.
Unlike Oil Red O and Nile Red, the Lipid Droplet Assay Kit - Blue and Deep Red enable selective and easy measuring of LDs as per the enclosed protocol. The Staining Dye in this kit minimizes background due to more selective staining ability to LDs than Nile Red. In addition, the Loading Buffer solution contained in this kit maintains the health of the cells during assays. This kit can be used for live and fixed cells in flowcytometry analysis and plate reader assay.
Kit Contents
-
- LD05 Lipid Droplet Assay Kit - Blue Staining Dye -Blue x 1 Loading Buffer (10x) 6 ml x 1
-
- LD06 Lipid Droplet Assay Kit - Deep Red Staining Dye- Deep Red x 1 Loading Buffer (10x) 6 ml x 1
Storage Condition
Store at 0–5 ℃.
Required Equipment and Materials
- Dimethyl sulfoxide (DMSO)
- Medium or HBSS
- Micropipettes
- Microtube
Preparation of Solutions
Preparation of the DMSO stock solution
Add 100 μl of DMSO to a tube containing Staining Dye and dissolve it by pipetting. Store the DMSO stock solution at -20 ℃.
- Protect the DMSO stock solution from light.
- The DMSO stock soltuion is stable at -20 o C for up to a month.
Preparation of the Loading Buffer solution
Dilute the Loading Buffer (10×) 10 times using double-deionized water.
- Please use the diluted solution on the same day of preparation.
Preparation of the working solution
Dilute the DMSO stock solution 200-fold with the Loading Buffer solution to prepare a working solution.
- Please use the diluted solution on the same day of preparation.
General Protocol
The dye staining
- Prepare cells for the assay.
- Discard the supernatant and wash the cells twice with HBSS.
- Add an appropriate volume of the working solution to the wells.
- Incubate the cells at 37 °C for 1–2 h.
- Discard the supernatant and wash the cells twice with HBSS.
- Add HBSS and detect the cells using flow cytometer or plate reader
- Please refer recomended filter settings bellow.
Use of other filters may cause fluorescence signal decreasing
-
Table 1 Suitable filters for microplate assay Lipid Droplet Assay Kit - Blue No. Excitation filter Emission filter 1 360 nm (20) 430 nm (35) 2 360 nm (20) 460 nm (20) 3 380 nm (20) 430 nm (35) 4 380 nm (20) 460 nm (20) Lipid Droplet Assay Kit - Deep Red No. Excitation filter Emission filter 1 590 nm (20) 635 nm (35) 2 590 nm (20) 670 nm (25) 3 620 nm (10) 670 nm (25)
-
Table 2 Suitable filters for flow cytometry Lipid Droplet Assay Kit - Blue Excitation laser Emission filter 405 nm 425-475 nm Lipid Droplet Assay Kit - Deep Red Excitation laser Emission filter 640 nm 650-670 nm
Usage Examples
Lipid droplet detection of triacsin C- or oleic acid-treated HepG2 cells.
- HepG2 cells (5×103 cells/well) in DMEM (10% fetal bovine serum, 1% penicillin-streptomycin) were seed on a 96-well black plate and were cultured at 37 °C in a 5% CO2 incubator overnight.
- The cells were washed twice with HBSS.
- DMEM (10% fetal bovine serum, 1% penicillin-streptomycin) containing 200 μmol/l oleic acid or 5 μmol/l triacsin C was added, and cultured at 37 °C in a 5% CO2 incubator overnight.
- The cells were washed twice with HBSS.
- The working solution was added to each well, and then the cells were cultured at 37 °C for 2 h in a 5% CO2 incubator.
- The cells were washed twice with HBSS.
- HBSS was added, and the cells were measured using a plate reader.
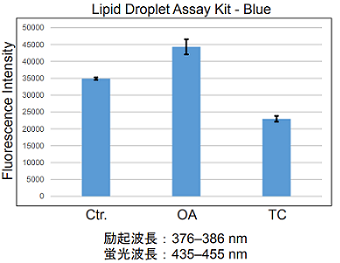
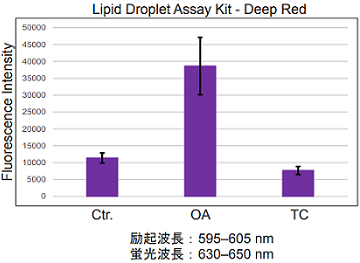
Fig. 1 Microplate assay of lipid droplets in HepG2 cells
Lipid droplet detection of triacsin C- or oleic acid-treated HeLa cells.
- HeLa cells (2.5×105 cells/ml) in MEM (10% fetal bovine serum, 1% penicillin-streptomycin) containing 200 μmol/l oleic acid or 5 μmol/l triacsin C were seeded on a 6-well plate and were cultured at 37 °C in a 5% CO2 incubator overnight.
- The cells were washed twice with HBSS.
- The working solution was added to each well, and then the cells were cultured at 37 °C for 2 h in a 5% CO2 incubator.
- The cells were washed twice with PBS.
- The cells were tripsinized.
- The supernatant was discarded, and HBSS was added.
- The cells were analyzed using a flow cytometer.
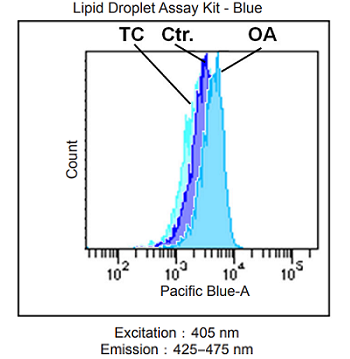
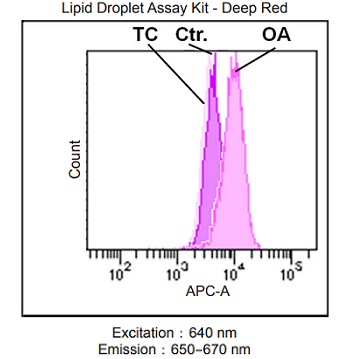
Fig. 2 Flow cytometric analysis of lipid droplets in HeLa cells
References
- Fujimoto, T. et al., Histochem Cell Biol., 2008, 130(2), 263–279.
- Singh, R. et al., Nature, 2009, 458(7242), 1131–1135.
- Yokoyama, M. et al., Cell Reports, 2014, 7(5), 1691–1703.
- Oka, M. et al., Cells, 2019, 8(4), 373
LD05_LD06: Lipid Droplet Assay Kit - Blue / Deep Red
Revised Jun., 22, 2023


 Hidden sections will not be printed.
Hidden sections will not be printed.