General Information
Lipid droplets (LDs) are composed of neutral lipids such as triacylglycerol and cholesteryl esters surrounded by a phospholipid monolayer, and are found not only in adipocytes1) but also ubiquitously in eukaryotic organisms. Although LDs were originally considered to be a type of lipid storage machinery, a recent study has shown that LDs play an important role in regulating lipid metabolism, autophagy2) and cellular senescence.3) Lipi probes are small molecules that emit strong fluorescence in a hydrophobic environment such as LDs, which can be observed without any washing steps after staining with Lipi probes.4)
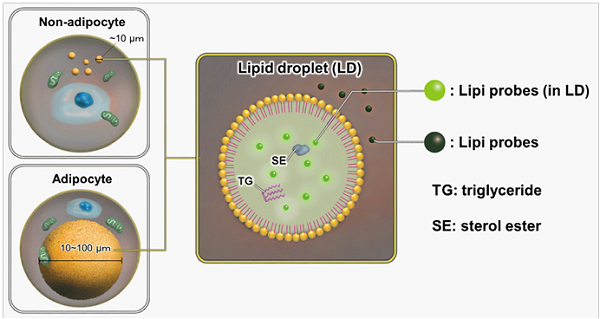
Figure 1. Staining mechanism of Lipi probes
Contents
| LD01 Lipi-Blue | 10 nmol x 1 |
| LD02 Lipi-Green | 10 nmol x 1 |
| LD03 Lipi-Red | 100 nmol x 1 |
| LD04 Lipi-Deep Red | 10 nmol x 1 |
- The material supplied for each dye is sufficient for 50 tests when a 35 mm dish is used. (final concentration of Lipi-Blue Lipi-Green, and Lipi-Deep Red: 0.1 μmol/l, Lipi-Red: 1 μmol/l)
Storage Condition
| LD01 | Store in a cool and dark place. |
| LD02 | Store in a cool and dark place. |
| LD03 | Store in a cool and dark place. |
| LD04 | Store in a cool and dark place. |
Required Equipment and Materials
- Dimethyl sulfoxide (DMSO)
- PBS
- Micropipettes
Fluorescent Properties
Fluorescent properties of Lipi probes
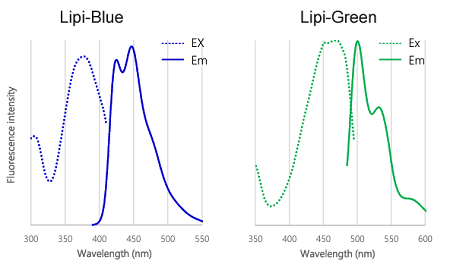
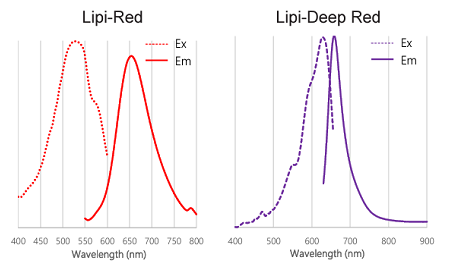
Figure 2. Excitation and emission spectra of Lipi-Blue, Lipi-Green, Lipi-Red, and Lipi-Deep Red
Preparation of Solutions
Preparation of Lipi probe DMSO stock solution
- Lipi-Blue 0.1 mmol/l DMSO stock solution: Add 100 μl of DMSO to a tube of Lipi-Blue and dissolve by vortex mixer.
- Lipi-Green 0.1 mmol/l DMSO stock solution: Add 100 μl of DMSO to a tube of Lipi-Green and dissolve by vortex mixer.
- Lipi-Red 1 mmol/l DMSO stock solution: Add 100 μl of DMSO to a tube of Lipi-Red and dissolve by vortex mixer.
- Lipi-Deep Red 0.1 mmol/l DMSO stock solution: Add 100 μl of DMSO to a tube of Lipi-Deep Red and dissolve by vortexing.
- Store the DMSO stock solution at -20 °C. The DMSO stock solution is stable at -20 °C for 1 month.
- Lipi-Blue is difficult to see because it is present in a small amount and is a colorless foam. Please prepare the Lipi-Blue DMSO stock solution carefully by vortexing with DMSO as described in the protocol.
Preparation of Lipi probe working solution
- Lipi-Blue working solution: Dilute the DMSO stock solution with PBS or serum-free medium to prepare 0.1–0.5 μmol/l working solution.
- Lipi-Green working solution: Dilute the DMSO stock solution with PBS or serum-free medium to prepare 0.1–0.5 μmol/l working solution.
- Lipi-Red working solution: Dilute the DMSO stock solution with PBS or serum-free medium to prepare 1–5 μmol/l working solution.
- Lipi-Deep Red working solution: Dilute the DMSO stock solution with PBS or serum-free medium to prepare 0.1 μmol/l working solution.
- Use the working solution within the same day of preparation.
- Serum-containing medium can also be used instead of serum-free medium.
General Protocol
- Seed cells on a dish for assay. Culture the cells at 37 oC overnight in a 5% CO2 incubator.
- Remove the culture medium and wash the cells with PBS twice.
- Add the Lipi series working solution and incubate at 37 oC for 30 minutes in the 5% CO2 incubator.
- When using epifluorescence microscope, replace the working solution with a culture medium or a buffer to reduce the fluorescence background.
- Observe the sample under a fluorescence microscope.
- Following filter sets are recommended.
Lipi-Blue: Excitation 405 nm, Emission 450–500 nm
Lipi-Green: Excitation 488 nm, Emission 500–550 nm
Lipi-Red: Excitation 561 nm, Emission 565–650 nm
Lipi-Deep Red: Excitation 640 nm, Emission 650–700 nm - If no fluorescent signal was observed, please try followings.
1.Increase the magnification of the fluorescence microscope in case the lipid droplets are small.
2.Increase the incubation time by 1–2 h.
3.Increase the reagent concentration up to 1 μmol/l for Lipi-Blue and Lipi-Green, 10 μmol/l for Lipi-Red, and 0.5 μmol/l for Lipi-Deep Red.
* When the reagent concentration is increased, it may occur a high background.
4. Prepare lipid droplet-containing cells as a positive control for comparison with the samples. The positive control can be prepared by incubating cells with a 200 μmol/l oleic acid-containing culture medium overnight.
Usage Examples
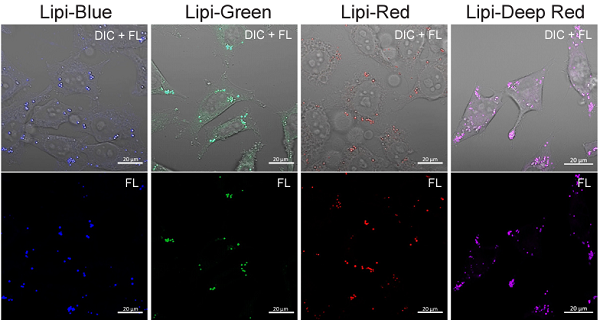
Induction of LDs formation using oleic acid (HeLa cells)
- HeLa cells were seeded on a μ-slide 8-well plate and cultured at 37 °C overnight in a 5% CO2 incubator.
- The supernatant was removed and the cells were washed twice with serum-free medium.
- Oleic acid (200 μmol/l)-containing medium (DMEM/10% FBS/1% PBS) was added to the each well, and the cells were cultured at 37 °C overnight in a 5% CO2 incubator.
- The supernatant was removed and the cells were washed twice with serum-free medium.
- The lipi working solution was added and the cells were incubated at 37 °C for 30 min in a 5% CO2 incubator.
- The cells were observed using a fluorescence microscope.
|
|
|
|
・Lipi-Blue ・Lipi-Green |
・Lipi-Red ・Lipi-Deep Red Scale bars: 20 μm |
Figure 3. Fluorescent images of oleic acid treated HeLa cells
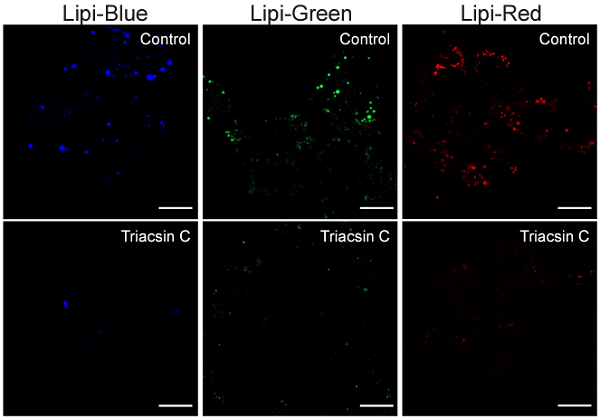
Inhibition of LDs formation using Triacsin C (HepG2 cells)
- HepG2 cells were seeded on a μ-slide 8-well plate and cultured at 37 °C overnight in a 5% CO2 incubator.
- The supernatant was removed and the cells were washed twice with serum-free medium.
- Triacsin C prepared with serum-containing medium (5 μmol/l) was added to the each well, and the cells were cultured at 37 °C overnight in a 5% CO2 incubator.
- The supernatant was removed and the cells were washed twice with serum-free medium.
- Lipi working solution was added and the cells were incubated at 37 °C for 30 min in a 5% CO2 incubator.
- The cells were observed using a fluorescence microscope.
|
|
|
|
・Lipi-Blue ・Lipi-Green |
・Lipi-Red Scale bars: 20 μm |
|
|
Figure 4. Fluorescent images of Triacsin C treated HepG2 cells
References
- Fujimoto, T. et al., Histochem Cell Biol., 2008, 130(2), 263–279.
- Singh, R. et al., Nature, 2009, 458(7242), 1131–1135.
- Yokoyama, M. et al., Cell Reports, 2014, 7(5), 1691–1703.
- Tatenaka, Y. et al., Biochemistry., 2019, 58(6), 499-503.
LD01_LD02_LD03_LD04: Lipi-Blue / Green / Red / Deep Red
Revised Jun., 21, 2023


 Hidden sections will not be printed.
Hidden sections will not be printed.