General Information
More than 10 years have passed since Stockwell et al. discovered ferroptosis, which is caused by an iron ion-dependent accumulation of lipid peroxides. It has become clear that ferroptosis is a form of cell death, in which free iron (Fe2+) generates reactive oxygen species (ROS) through the Fenton reaction1), which in turn oxidize lipids. More recently, it has been reported that lipid oxidation damages lysosomal membranes and causes iron to leak from lysosomes, thereby spreading it to other cellular organelles2). Lyso-FerroRed can detect intracellular lysosomal free iron (Fe2+). Lyso-FerroRed penetrates the cell membrane and selectively reacts with Fe2+ in lysosomes, emitting strong fluorescence. Reaction between Lyso-FerroRed and Fe2+ is irreversible and differs from those based on the detection principle of calcium iron probes such as Fluo 3. Lyso-FerroRed has no chelating ability.
Content
Lyso-FerroRed 35 nmol×1
Storage Condition
Store in a cool and dark place.
Required Equipment and Materials
– Fluorescence microscope, fluorescence plate reader or flow cytometer
– Incubator (37℃)
– Micropipettes (100–1000 μl, 20–200 μl, 0.5–10 μl)
– Microtubes
– Medium or Hanks' Balanced Salt Solution (HBSS)
Precaution
Lyso-FerroRed is difficult to see because it is present in small amounts and appears as a colorless foam.
Centrifuge the tube briefly before opening the cap because the content may be adhered to the tube wall or the inside of the cap.
Please refer to Table 1 for suitable fluorescence wavelengths for each application.
Table 1. Recommended filter settings of the Lyso-FerroRed
| Applications | Fluorescence microscope | Fluorescence plate reader | Flow cytometer |
| Measurement wavelength |
・Confocal microscope Ex/Em: 564/565–620 nm ・Fluorescence microscope TRITC filter |
Excitation: 540–560 Emission: 570–590 nm |
Ex/Em: 564/565–620 nm |
Preparation of Solutions
Preparation of 1 mmol/l Lyso-FerroRed DMSO stock solution
Add 35 μl of DMSO to a tube containing 35 nmol of Lyso-FerroRed and dissolve the solution by pipetting to prepare a 1 mmol/l Lyso-FerroRed DMSO stock solution.
- The 1 mmol/l Lyso-FerroRed DMSO stock solution is stable at −20℃ for 1 month.
Preparation of 1 μmol/l Lyso-FerroRed working solution
Dilute the 1mmol/l Lyso-FerroRed DMSO stock solution 1:1000 in serum-free medium or HBSS to prepare the 1 μmol/l Lyso-FerroRed working solution.
- The working solution cannot be stored and must be prepared freshly each day.
- Refer to Table 2 for the preparation of the working solution by vessel type.
Table 2. Required amount of the working solution by vessel type
| Vessel | 35-mm dish | ibidi 8-well plate | 96-well black plate (clear bottom) |
| Appropriate amount | 2 ml | 200 μl/well | 100 μl/well |
General protocol
- Seed cells on a dish. Culture the cells at 37°C overnight in a 5% CO2 incubator.
- Discard the supernatant and wash the cells twice with the serum-free medium or HBSS.
- Add serum-free medium or HBSS containing stimulation agents and incubate the cells as in step 1 for an appropriate time.
- Discard the supernatant and wash the cells twice with serum-free medium or HBSS.
- Discard the supernatant, add an appropriate volume of Lyso-FerroRed working solution to the dish, and incubate at 37°C for 30 minutes in a 5% CO2 incubator.
- Discard the working solution and wash the cells twice with HBSS.
- Observe the cells under a fluorescence microscope.
Usage example
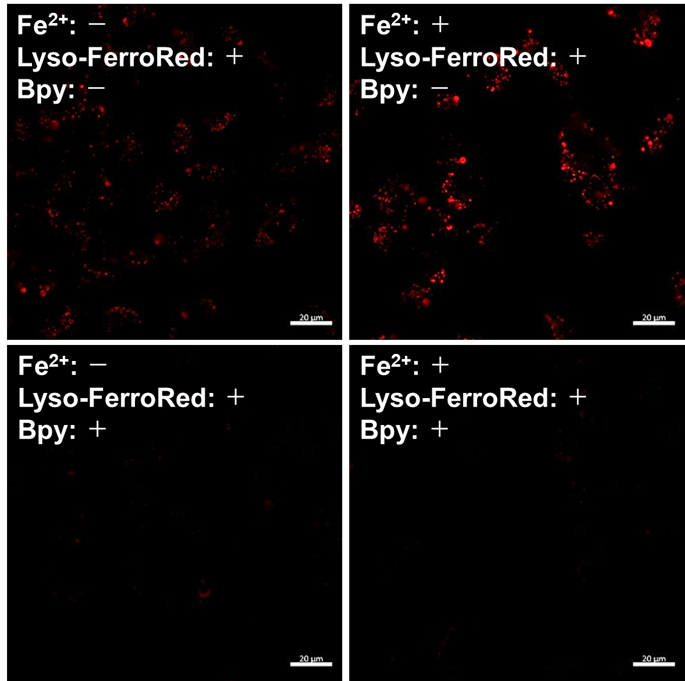
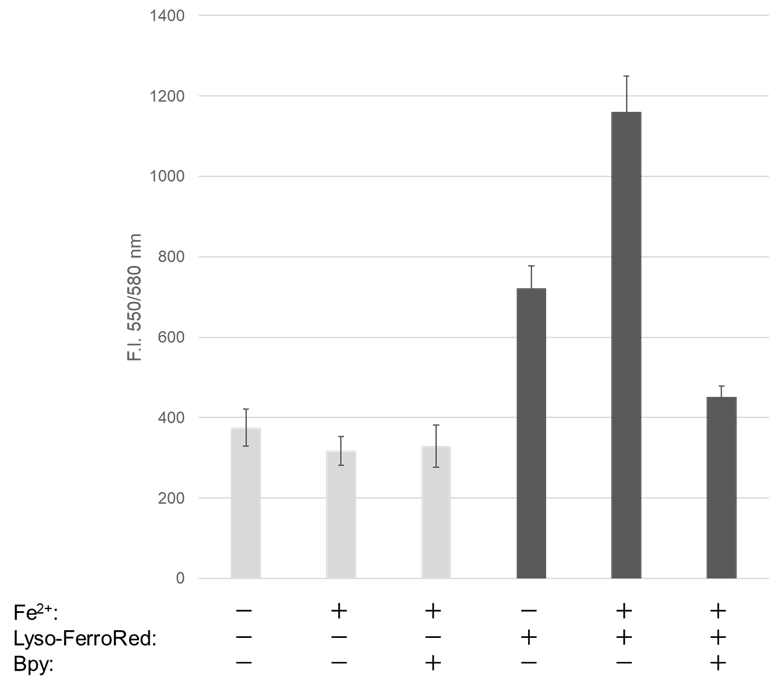
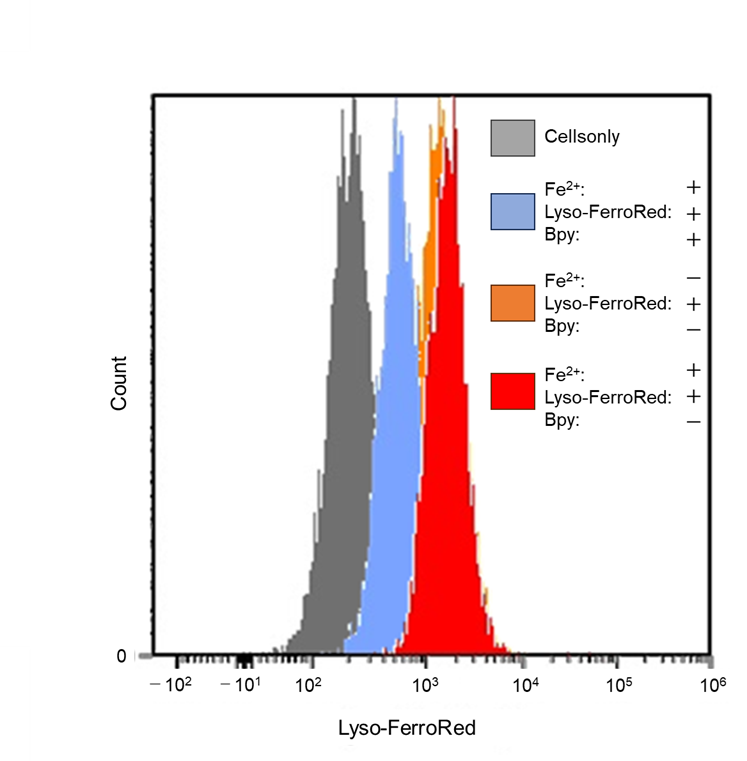
Detection of lysosomal Fe iron in HT-1080 cells treated with Fe(NH4)2(SO4)2・6H2O.
- HT-1080 cells in MEM (supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin) were seeded into vessels and incubated overnight at 37℃ in 95% air/5% CO2.
- 96-well black plate, 1×104 cells/well; ibidi 8-well plate, 3×104 cells/well; 35-mm dish, 3×105 cells/well.
- After the culture medium was removed, the cells were washed twice with serum-free medium, and the vessels were refilled with serum-free MEM.
- 96-well black plate, 100 μl/well; ibidi 8-well plate, 200 μl/well; 35-mm dish, 2 ml/well.
- Ammonium iron (II) sulfate (10 mmol/l) was prepared in purified water.
- Ammonium iron (II) sulfate (10 mmol/l stock solution) was added to the wells to give a final concentration of 100 µmol/l. To mix the ammonium iron (II) sulfate and serum-free medium, the entire medium was pipetted up from the wells and then immediately pipetted back once.
- 96-well black plate, 1 µl of 10 mmol/l ammonium iron (II) sulfate was added per well; ibidi 8-well plate, 2 µl added/well; 35-mm dish, 20 µl added/well.
- When adding 10 mmol/l ammonium iron (II) sulfate to wells, please follow step 4 exactly as described. Do not add pre-prepared 100 μmol/l ammonium iron (II) sulfate to cells. It may result in the precipitation of ammonium iron (II) sulfate during the experiment.
- The cells were incubated at 37℃ for 30 minutes in a 5% CO2 incubator.
- The supernatant was removed, and the cells were washed twice with serum-free medium.
- Lyso-FerroRed (1 μmol/l) and 2,2'-bipyridyl (Bpy; 1 mmol/l) were added to the cells in serum-free medium, and then the cells were incubated at 37℃ for 30 minutes in a 5% CO2 incubator.
- The supernatant was removed, and the cells were washed twice with HBSS. The vessels were refilled with HBSS.
- Fluorescence signals were measured/observed using a plate reader, flow cytometer, and confocal laser microscope.
 |
 |
 |
|
Detection: Confocal laser microscope |
Detection: Plate reader |
Detection: Flow cytometer Ex/Em: 565/565–617 nm |
Figure 1. Fluorescence signals from HT-1080 cells detected with a confocal laser microscope, plate reader, and flow cytometer
Reference
1)Brent R. Stockwell et al., Cell, 2012, 149, 1060.
2)K. Yamada et al., Nature Communications, 2025, 16, 2554.
Frequently Asked Questions / Reference
L270: Lyso-FerroRed
Revised Aug., 19, 2025


 Hidden sections will not be printed.
Hidden sections will not be printed.

