General Information
Lactate is a metabolite of glycolysis that is one of the main metabolic pathways in cells, and is known to be a biomarker for muscular fatigue and hyperlactacidemia. It also serves as a marker for monitoring the changes of intracellular metabolic pathways. In addition, recent metabolomic study suggests that lactate contributes as a major carbon source in the TCA cycle of tissues and cancer cells1).
Lactate Assay Kit-WST enables quantitation of lactate produced by glycolysis. This kit has been optimized to quantitate lactate in cell culture supernatant by measuring the absorption derived from a colorimetric reaction of WST. This kit is formated for 96-well microplate assays with a detection sensitivity limit of 0.02 mmol/l lactate.
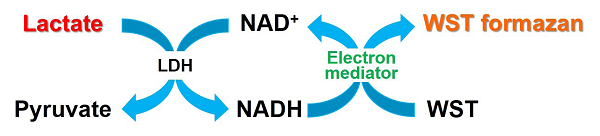
Fig. 1 Principle of Lactate Assay Kit-WST
Kit Contents
| 50 tests | 200 tests | |
| Dye Mixture | x1 | x1 |
| Lactate Standard (10 mmol/l) | 150µl x1 | 600 µl x1 |
| Enzyme Solution | 12 µl x1 | 48 µl x1 |
| Assay Buffer | 5.5 ml x1 | 11 ml x2 |
| Reconstitution Buffer | 550 µl x1 | 2.2 ml x1 |
Storage Condition
Store at 0-5℃
Required Equipment
- Microplate reader (450 nm filter)
- 96-well microplate
- Incubator (37°C)
- 20-200 µl multichannel pipette
- 20 µl, 200 µl, 1000 µl micropipettes
Precautions
- Equilibrate the kit to room temperature prior to use.
- Pipete the Enzyme Solution before use to obtain the homogenous mixture since an enzyme is suspended in a liquid.
- Triplicate measurement per sample is recommended to obtain accurate data.
- Since the enzymatic reaction starts immediately after the addition of Working solution to a well, use a multichannel pipette to minimize the experimental error from time lag in pipetting.
- Please prepare samples with different dilution rate and determine the suitable dilution rate to be ranging from 0 to 1 mmol/l.
- A glass bottle and an aluminum cap are used as a package of Dye Mixture. Use protective gloves with cautious in handling.
- This kit is designed for measuring cell culture supernatant samples. For measuring a concentration of intracellular lactate, use 0.1% Triton solution for preparation of cell lysate and Lactate standard solution.
Preparation of Solutions
Preparation of Dye Mixture stock solution
Add all Reconstitution Buffer to a Dye Mixture vial. Close the cap and dissolve the contents completely.
- Transfer the Dye Mixture stock solution to the vial of the Reconstitution Buffer and store it at 0-5℃ with protection from light.
Dye Mixture stock solution is stable for 4 months under these conditions.
Preparation of Working solution
- Add Dye Mixture stock solution to a conical tube and dilute it with Assay Buffer.
- Add Enzyme Solution to the solution prepared in step 1.
- Refer to Table 1.
- Working solution is light sensitive. Prepare the solution just before use and protect it from light by covering with aluminum foil. Please use up Working solution within that day.
| for 24 well | for 48 well | for 96 well | |
| Dye Mixture stock solution | 250 µl | 500 µl | 1 ml |
| Assay Buffer | 2.25 ml | 4.5 ml | 9 ml |
| Enzyme Solution | 5 µl | 10 µl | 20 µl |
General Protocol
1. Sample preparation
Prepare cell culture supernatant samples (Sample).
- Please prepare samples with different dilution rate and determine the suitable dilution rate to be ranging from 0-1 mmol/l.
Use double-deionized H2O (ddH2O) for diluting. - In case a medium contains serum, read the blank absorbance (serum containing medium) as background control and subtract its value from absorbance of each sample.
- Required sample amount is 20 µl for each well.
2. Preparation of Lactate standard solution
Mix 50 µl of 10 mmol/l Lactate Standard and 450 µl of ddH2O in a microtube to prepare a 1 mmol/l Lactate standard solution. Prepare the following Lactate standard solution by serial dilution with ddH2O: 1, 0.5, 0.25, 0.125, 0.0625, 0.0313, 0.0157 and 0 mmol/l (Fig. 2).
- For measuring a concentration of intracellular lactate, prepare Lactate standard solution with 0.1% Triton solution instead of ddH2O.
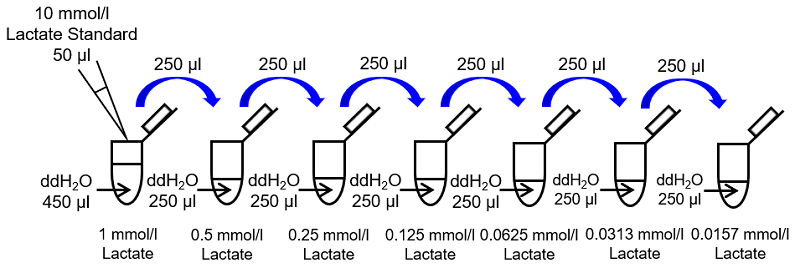
Fig. 2 Preparation of Lactate standard solution
3. Measurement
- Add 20 µl of Lactate standard solution and sample solutions to each well (Fig. 3).
- In order to obtain accurate data, we recommend triplicate measurement per sample.
- Add 80 µl of Working solution to each well.
- Since the enzymatic reaction starts immediately after the addition of Working solution to the well, use a multichannel pipette to minimize the experimental error from time lag in pipetting.
- Incubate the microplate at 37℃ for 30 minutes.
- Use a seal for the microplate to prevent evaporation of the solution during the incubation.
- Measure the absorbance at 450 nm by using a microplate reader.
- Determine the concentration of lactate in the sample using a calibration curve.
- If the original samples have been diluted for this assay, multiply the determined value and dilution rate.
-
1 2 3 4 5 6 A 0 mmol/l Lactate Sample 1 B 0.0157 mmol/l Lactate Sample 2 C 0.0313 mmol/l Lactate Sample 3 D 0.0625 mmol/l Lactate Sample 4 E 0.125 mmol/l Lactate Sample 5 F 0.25 mmol/l Lactate Sample 6 G 0.5 mmol/l Lactate Sample 7 H 1 mmol/l Lactate Sample 8 Fig. 3 An example of plate arrangement (n=3)
-
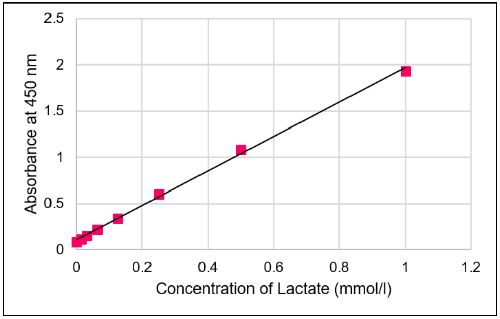
Fig. 4 Typical calibration curve of lactate
Experimental Example
Glycolysis inhibition by 2-deoxy-D-glucose
- HeLa cells (1×104 cells/well, MEM containing 10% fetal bovine serum and 1% penicillin-streptomycin) were seeded in a 96-well microplate and cultured overnight in a 5% CO2 incubator.
- After the removal of supernatant, 100 µl of medium containing 2-deoxy-D-glucose was added.
- The cells were cultured overnight in the 5% CO2 incubator.
- After the incubation, 20 µl of the cell culture supernatant was transferred to a 1.5-ml microtube and diluted 8 times with ddH2O to prepare the sample solution, and then 20 µl of the sample solution was added to each well.
- Working solution (80 µl) was added to each well.
- The 96-well microplate was incubated at 37℃ for 30 minutes.
- The absorbance at 450 nm was measured by using a microplate reader, and the concentration of lactate in the sample was determined using a calibration curve.
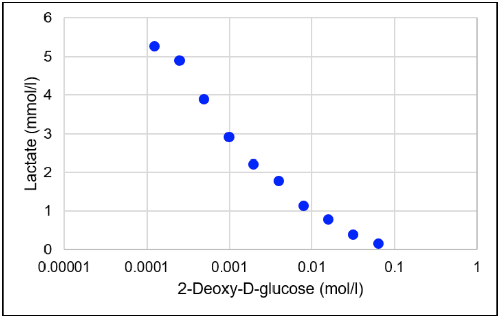
Fig. 5 Glycolysis inhibition by 2-deoxy-D-glucose
Lactate concentration decreased with increasing concentrations of 2-deoxy-D-glucose (one of the glycolysis inhibitors).
Reference
1) S.Hui, et al., Nature, 2017, 551, 115.
Frequently Asked Questions / Reference
L256: Lactate Assay Kit-WST
Revised May., 24, 2023


 Hidden sections will not be printed.
Hidden sections will not be printed.