Important notice
This is the manual for "Type2".
Please check the product packaging below and use the manual for each product.
|
|
||||||
General Information
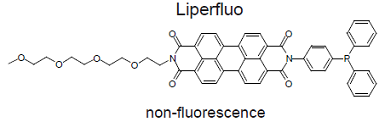
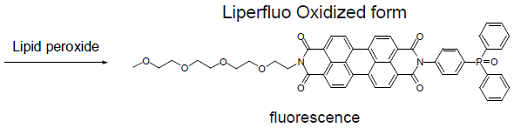
Liperfluo is used for lipid peroxide detection, and it emits intense fluorescence by the lipid peroxide specific oxidation in organic solvents such as ethanol. Since the excitation and emission wavelengths of the oxidized form of Liperfluo are 524 nm and 535 nm, respectively, both photo-damage against a sample and an auto-fluorescence from the sample can be minimized. Due to the introduction of a tetraethyleneglycol group at the one-end of diisoquinoline ring, the dispersibility of the molecule in an aqueous buffer is improved. Though Liperfluo oxidized form is almost non-fluorescent in an aqueous media, it emits fluorescence in lipophilic sites such as in cell membranes. Therefore it can be easily applied for a lipid peroxide imaging by fluorescence microscopy or lipid peroxide analysis by flow cytometry for a living cell.
Kit Contents
| Liperfluo | 50 μg x 5 |
Storage Conditions
Store at 0-5°C and protect from light
Preparation of Solutions
Preparation of a 1 mmol/l Liperfluo DMSO solution
- Add 60 μl of DMSO to a tube containing Liperfluo.
- Protect the tube from light with alumininum foil and vortex it for about 2 minutes.
Once prepared, Liperfluo DMSO solution should be protected from light and used within 1 day.
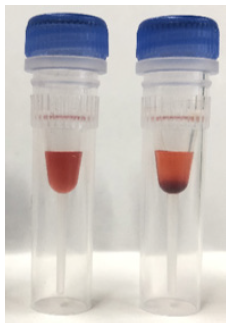 |
Left image : Liperfluo (completely dissolved) Right image : Liperfluo (dissolving is not enough) |
- If Liperfluo solids are present at the bottom of the tube, extend the vortexing.
Preparation of a Liperfluo Working solution
The concentration of Liperfluo for optimal staining will vary depending on the experimental conditions.
Follow the procedures below.
For microscopic imaging
Dilute the 1 mmol/l Liperfluo DMSO solution with serum-free culture medium to prepare Liperfluo working solution.
For flow cytometry
Add the 1 mmol/l Liperfluo DMSO solution directry to cell suspension to be stained.
General Protocol
Detection of lipid peroxide in the cells
- Seed the cells in a dish or plate.
- Remove the supernatant and wash the cells with serum-free culture medium once.
- Add an appropriate volume of Liperfluo working solution and incubate at 37°C for 30 minutes.
- Optimize the final concentration of Liperfluo because the optimal concentration of Liperfluo depends on experimental conditions and other factors.
- Remove the supernatant and wash the cells with serum-free culture medium twice.
- Observe the cells under a fluorescence microscope or analyze the cells using a flow cytometer.
Experimental Example
Detection of lipid peroxide by confocal microscopy
- HeLa cells in MEM (containing 10% fetal bovine serum) were seeded (3.0×104 cells/well) in a 8 well slide (ibidi) and cultured overnight at 37°C in a 5% CO2 incubator.
- Removed the supernatant and the cells were washed with serum-free culture medium once.
- Serum-free culture medium (200 μl) containing 1 μmol/l Liperfluo was added to well and the cells were incubated at 37°C for 30 minutes in a 5% CO2 incubator.
- The medium was removed and the cells were washed with 200 μl of HBSS twice.
- HBSS (200 μl) containing 500 μmol/l t-BHP (tert-buthyl hydroperoxide) was added to the well and the cells were incubated for 60 minutes at 37°C in a 5% CO2 incubator.
- The cells were observed under a confocal fluorescence microscope. (Excitation: 488 nm, Emission: 500-550 nm).
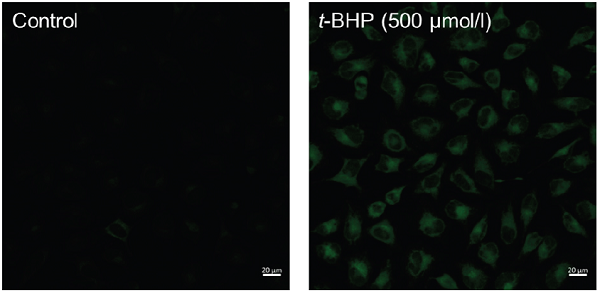
Fluorescence images of lipid peroxidation in HeLa cells.
Detection of lipid peroxide by Flow Cytometry
- HeLa cells in MEM (containing 10% fetal bovine serum) were seeded (1.0×105 cells/well) in a 6 well plate (Thermo) and cultured overnight at 37°C in a 5% CO2 incubator.
- Removed the supernatant and the cells were washed with serum-free culture medium once.
- Serum-free culture medium (2 ml) containing 1 μmol/l Liperfluo was added to well and the cells were incubated at 37°C for 30 minutes in a 5% CO2 incubator.
- The medium was removed and the cells were washed with 2 ml of HBSS twice.
- HBSS (2 ml) containing 500 μmol/l t-BHP (tert-buthyl hydroperoxide) was added to the well, and the cells were incubated for 60 minutes at 37°C in a 5% CO2 incubator.
- The cells were harvested by trypsinization and collected into a microtube with culture medium.
- The supernatant was replaced with HBSS and the cells were analyzed using a flow cytometer(Excitation: 488 nm, Emission: 515-545 nm).
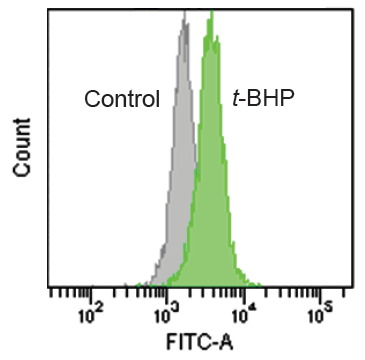
Quntification of lipid peroxide with Liperfluo.
Detection of lipid peroxide induced by ferroptosis with erastin (inhibitor for cystine transporter)
- A549 cells in DMEM (containing 10% fetal bovine serum) were seeded (1.0×105 cells/well) in a 8 well slide (ibidi) and cultured overnight at 37°C in a 5% CO2 incubator.
- The DMEM was removed and 200 μl of serum-free culture medium containing 50 μmol/l erastin was added to well.
- The cells were incubated at 37°C overnight in a 5% CO2 incubator.
- The medium was removed and the cells were washed with 200 μl of HBSS twice.
- HBSS (200 μl) containing 5 μmol/l Liperfluo was added to well and the cells were incubated at 37°C for 30 minutes in a 5% CO2 incubator.
- The supernatant was removed and the cells were washed with 200 μl of HBSS twice.
- The cells were observed under a confocal fluorescence microscope. (Excitation: 488 nm, Emission: 500-550 nm)
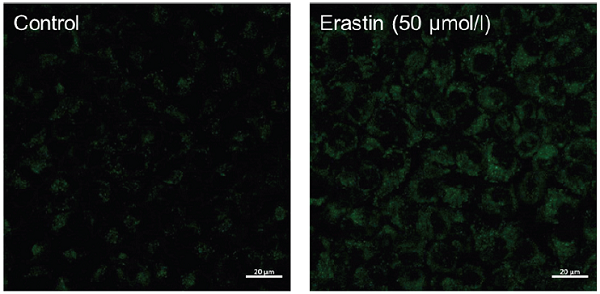
Fluorescence images of lipid peroxidation in A549 cells stimulated by erastin.
Stronger fluorescent signal of Liperfluo was observed in the ferroptosis induced sample
Fluorescence Property
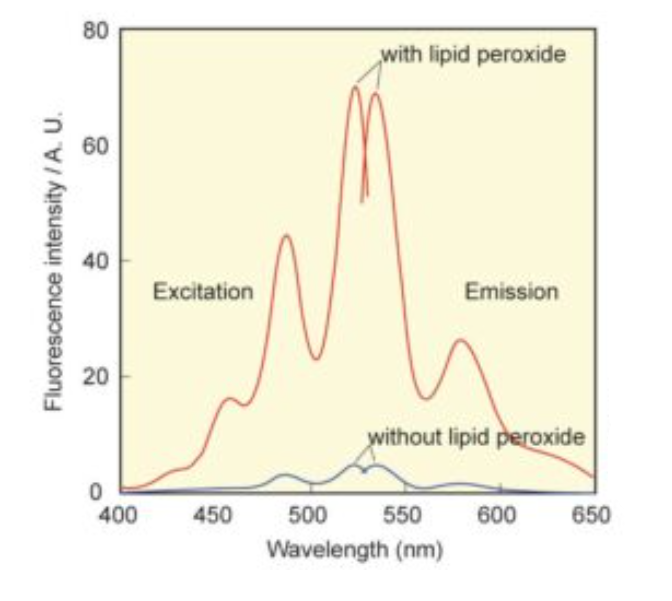
Excitation and emission spectra of Liperfluo with or without lipid peroxide in ethanol.
Frequently Asked Questions / Reference
L248: Liperfluo
Revised Feb., 21, 2024


 Hidden sections will not be printed.
Hidden sections will not be printed.