General Information
HilyMax is a newly developed gene transfection reagent. The transfection reagent forms a liposome to be used on highly efficient gene transfection to a wide variety of cells. HilyMax can be utilized to siRNA transfection as well. Since serum in the growth medium does not interfere with the transfection using HilyMax, no exchange of the media during the transfection is required. HilyMax does not contain any biological components that might interfere with the transfection.
Kit Contents
| HilyMax Reagent | x 1 |
| Lipoform Buffer | 1.0 ml x 1 |
Storage Condition
Kit: Store at 0-5oC.
Reconstituted HilyMax solution: Store at -20oC. The reconstituted solution is stable at -20oC for 6 months.
*For frequent usage of the reconstituted HilyMax solution, store at 0-5oC and use the solution within 1 month.
.png) |
After thawing the freezed HilyMax solution, mix it well by vortex or pipetting. Freeze/thaw cycle does not affect the transfection efficiency. |
Preparation of HilyMax
Add Lipoform Buffer 1.0 ml to HilyMax Reagent and mix the solution well by vortex for 30 seconds or pipetting until the reagent is dissolved completely.
.png) |
Check if HilyMax reagent is completely dissolved. If the insoluble form remains in the solution, continue the vortex or pipetting until the reagent is completely dissolved. |
Precautions
Transfection efficiency varies significantly with types and densities of cells. We strongly recommend to search or seek a transfection condition for your best performance with HilyMax (see “Optimization Protocol” on reverse page).
The optimized protocol for following 18 cell lines are available at www.dojindo.com.
A549 cell, Caco2 cell, CHO cell, COS-7 cell, HEK293 cell, HeLa cell, HepG2 cell, K562 cell, L6 cell, LNCap cell, MCF7 cell, MDCK cell,
Neuro2a cell, NIH3T3 cell, PC3 cell, PC12 cell, U937 cell, Vero cell are available. (Jan. 10, 2011)
General Protocol
Transfection procedure for a 24-well plate
1. Cell preparation
- Adherent cells
Prepare cell culture plate with 50-90% cell density.a)
- Non-adherent cells
Prepare 1.0-10 x 105 cells/ml cell suspension with cell culture medium.b)
Add 0.5 ml of cell suspension to each well.
.png)
2. DNA-HilyMax complex formationc)
-
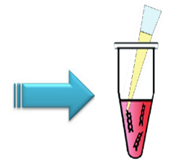
Step 1.
Add 30 µl of serum-free mediumd) to a plastic tube.Step 2.
Add plasmid DNA (0.5-1.5 µg)e) to the tube and mix by pipetting.
-
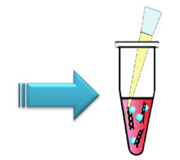
Step 3.
Add HilyMax to prepare DNA (µg):HilyMax (µl)=1:2-1:7 and mix by pipetting.Step 4.
Incubate the tube at room temperature for 15 minutes.f)
.png)
- 3. Addition of complex to cells
Add DNA-HilyMax complex to cell culture well.
- 4. Transfection
Incubate the plate at 37oC in a CO2 incubator for 4 hours.g) Change the medium and continue incubation for 18-48 hours.
- 5. Downstream experiment
Reporter gene assay, protein isolation, protein analysis, etc.
a) 50-90% cell density means that 50-90% of the total surface area is covered with cells.
b) Serum does not interfere with the transfection.
c) The appropriate amounts of DNA and HilyMax for various vessels are indicated in Table 2.
d) Serum and antibiotics in the medium interfere with the DNA-HilyMax complex formation.
However Opti-MEM, DMEM, and MEM can be used for transfection.
e) Use purified plasmid DNA(A260/A280=1.7-1.9). The recommended concentration of DNA for transfection is 0.15-1.00 mg/ml.
f) Excess incubation time may cause a lower transfection efficiency.
g) A medium change increases the transfection efficiency and decreases the cytotoxicity for most of cell lines.
Optimization Protocol
Optimization of transfection for a 24-well plate
The transfection efficiency may increase 2-5 times by executing the following optimization step.
The amount of DNA and HilyMax, cell density, and medium change are the most significant factors to optimize your transfection efficiency. We strongly recommend you carry out optimization protocol for your first trial.
.png)
Figure 1. Example of plate arrangement for optimization
Value in each well indicates the volume of HilyMax.
A: Without Medium Change.
B: With Medium Change 4 hours after Transfection.
- Transfection Procedure (for a 24-well plate)
- Prepare cell culture plate with 50% and 80% cell density.
- Add serum-free medium*1 (30 µl) to a plastic tube.
- Add plasmid DNA*2 (0.5-1.5 µg) to the tube and mix.
- Add HilyMax*2 (1.5-10.5 µl) to the tube and mix.
- Incubate the tube at room temperature for 15 minutes.
- Add DNA-HilyMax complex to cell culture well (step 1).
- Incubate at 37oC in CO2 incubator in 18-48 hours incubation.
- Change the medium for the row B after 4 hours incubation.
- Measure the transfection efficiency.
If you are using other size of vessels, change the amount of medium, DNA, and HilyMax.
- Refer to Table 2 (Serum-Free Medium).
- Multiply the coefficient in Table 1 by the amount of DNA and HilyMax.
| 96-well plate : x 0.2 | 12-well plate : x 2 | 6-well plate (35-mm dish) x 4 |
| 60-mm dish : x 8 | 100-mm dish : x 24 |
Transfection Condition for Various Vessels
Table 2 shows suitable amount of medium, DNA, and mixing ratio of DNA and HilyMax solution against each vessel.
| Culture Vessel | Surface Area | Plating Medium | Serum-Free Medium | DNA | DNA(µg):HilyMax(µl) |
| 96-well | 0.3 cm2 | 0.1 ml | 10 μl | 0.1-0.3 μg | 1:2-1:7 |
| 24-well | 1.9 cm2 | 0.5 ml | 30 μl | 0.5-1.5 μg | 1:2-1:7 |
| 12-well | 3.8 cm2 | 1.0 ml | 60 μl | 1.0-3.0 μg | 1:2-1:7 |
| 6-well | 9.2 cm2 | 2.0 ml | 120 μl | 2.0-6.0 μg | 1:2-1:7 |
| 35-mm | 8.0 cm2 | 2.0 ml | 120 μl | 2.0-6.0 μg | 1:2-1:7 |
| 60-mm | 21.0 cm2 | 5.0 ml | 300 μl | 5.0-15.0 μg | 1:2-1:7 |
| 100-mm | 58.0 cm2 | 15.0 ml | 900 μl | 15.0-45.0 μg | 1:2-1:7 |
Troubleshooting
Transfection efficiency is low.
- The volume of HilyMax is not enough. Increase the HilyMax volume.
- Cell density is too high. Reduce the cell density. The appropriate cell density for transfection is about 50-90%.
- HilyMax reagent is not dissolved completely. Please check that the HilyMax solution is homogeneous.
- Incubation time for the preparation of HilyMax and DNA complex is too long.
- Your culture medium for DNA-HilyMax complex formation contain serum and/or antibiotics. Please use serum and antibiotics free medium for the complex formation.
Toxicity is high. Most cells are died.
- Reduce the amount of DNA and/or HilyMax.
- Cell density is too low. Increase the cell density. The appropriate cell density for transfection is about 50-90%.
FAQ
Are there transfection conditions for specific cell lines available?
Please contact our customer service at info@dojindo.co.jp.
Does frequent freeze and thaw of HilyMax solution affect transfection efficiency?
No. Freeze/thaw process is repeatable without reducing the efficiency.
Frequently Asked Questions / Reference
H357: HilyMax
Revised Nov., 29, 2023


 Hidden sections will not be printed.
Hidden sections will not be printed.