General Information
Recent findings suggest that a decline in internal antioxidant capacity causes the onset of various diseases and health impairment. Consequently, interest in antioxidant rich foods has been recently increasing. Shimamura et al. improved the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay, a procedure for evaluating antioxidant capacities, and reported it may be applicable as a standard method to evaluate the antioxidant capacity of antioxidants.1)
The DPPH Antioxidant Assay Kit is based on the DPPH assay improved by Shimamura and enables quick and easy measurements of the antioxidant capacity of a sample. Using this kit, the antioxidant capacity is expressed as the Trolox equivalent antioxidant capacity (TEAC), a value calculated from the IC50 of the antioxidant and the IC50 of Trolox. The DPPH Reagent and Trolox Standard included in this kit only need to be dissolved prior to use. Moreover, the protocol for this kit supports a microplate assay format for the simultaneous analysis of multiple samples.
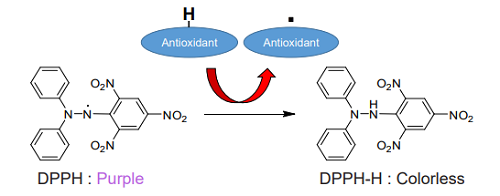
Figure 1. Principle of the DPPH Antioxidant Assay Kit
Kit Contents
| 100 tests | 500 tests | |
| DPPH Reagent | x 1 | x 5 |
| Trolox Standard | 1 mg x 1 | 1 mg x 5 |
| Assay Buffer | 11 ml x 1 | 55 ml x 1 |
Storage Conditions
- Store at 0–5oC
Required Equipment and Materials
-
- Ethanol (99.5%)
- 10 ml measuring flask
- Microplate reader (equipped with a filter around 517 nm)
- 96-well Microplate
-
- Incubator (25ºC)
- Micropipettes
- 20–200 μl Multichannel pipette
- Ultrasonic cleaner
Preparation of Solutions
Preparation of the DPPH working solution
- Add approximately 1 ml of ethanol to a tube of DPPH Reagent and sonicate for 60 seconds.
- Transfer all of the solution prepared in step 1 to a 10 ml measuring flask.
- Add another aliquot of approx. 1 ml of ethanol to the tube from step 1 and sonicate for 60 seconds.
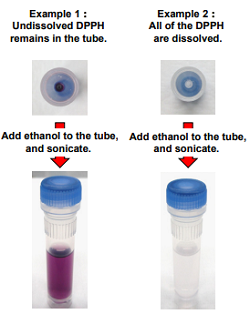
- Check the color of the solution and if the solution has a purple color as shown in the images on the below, repeat steps 2 and 3 until no color is observed.
- Please use sonication to dissolve the DPPH Reagent because it is difficult to dissolve.
- Undissolved DPPH may result in the variation of data.
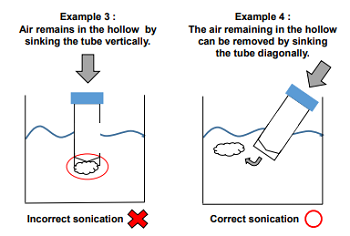
Please make sure that all of the DPPH is dissolved. - If air remains in the hollow of the tube, it is hard to dissolve the DPPH Reagent completely by sonication.
Please remove the air by sinking the tube of the DPPH Reagent diagonally into a ultrasonic cleaner.
- Make up to a final volume of 10 mL with ethanol.
- Prepare the DPPH working solution fresh each day.
Preparation of the Trolox Standard solution
- Add approximately 1 ml of ethanol to the Trolox Standard tube and completely dissolve the contents by vortexing or sonication.
- Transfer all of the solution prepared in step 1 to a 10 ml measuring flask and add ethanol to 10 ml.
- Dilute the 100 μg/ml Trolox Standard solution prepared in step 2 with ethanol to make 80, 60, 40, and 0 μg/ml solutions (Figure 2).
- Prepare the Trolox Standard solution fresh each day.
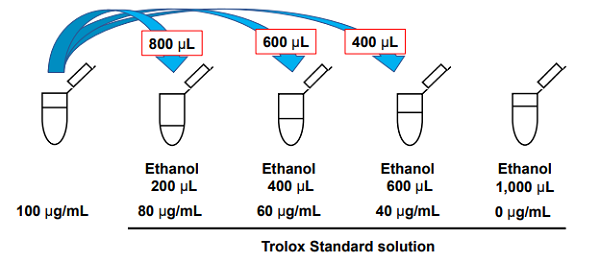
Figure 2. Preparation of the Trolox Standard solutions
Preliminary Experiment
·TEAC is calculated based on the IC50 values for the Trolox Standards and samples, defined as the concentration at which 50% of the DPPH-radicals are scavenged.
·To determine the IC50, the concentration range of each sample should be optimized as described below.
- Table 1. Amount of each solution to add to a well
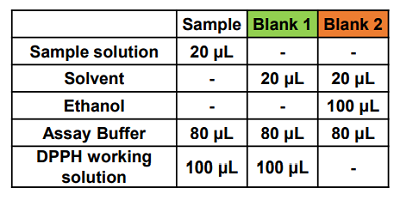
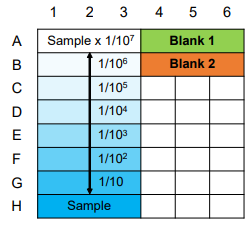
Figure 3. Example of a plate format for a preliminary experiment
- Blank 1: coloring without antioxidant, Blank 2: sample solvent blank.
- If the sample is highly colored, prepare a sample blank for each concentration.
Determination of the optimum concentration range
- Please follow the order of this protocol because it is optimised for an antioxidant assay.
- Prepare four or more concentrations for each sample with a 10-fold dilution from the highest concentration.
- Add 20 μl of each sample prepared in step 1 to the appropriate wells.
- Add 20 μl of solvent used for sample extraction or dilution to the wells of Blank 1 and Blank 2.
- To avoid any concentration change because of volatilization, move to step 4 immediately.
- Add 80 μl of Assay Buffer to each well.
- Add 100 μl of ethanol to the wells of Blank 2 and mix well by pipetting.
- Add 100 μl of DPPH working solution to the wells of the samples and Blank 1, and mix well by pipetting.
- Because the reaction starts immediately after the addition of the DPPH working solution, it should be dispensed with a multichannel pipette to minimize any time lag in pipetting.
- DPPH working solution is also used for the antioxidant capacity assays described later.
To avoid any concentration change because of volatilization, dispense only the required volume of DPPH working solution into a reservoir.
- Incubate the microplate at 25oC for 30 min in the dark.
- Measure the absorbance at 517 nm using a microplate reader.
- If the 517 nm filter is not available, measure the absorbance at 500 nm or greater (as close as possible to 517 nm).
- Calculate the inhibition ratio of the samples from the following equation:
Inhibition ratio of sample (%) = (ACS - AS)/ACS x 100 ACS :Blank 1 - Blank 2 AS :Absorbance of samples - Blank 2 or sample blank (in the case where the sample is highly colored) - Plot the inhibition ratio (y) against the sample concentration (x) and draw a regression line (y=ax+b).
- Determine the optimum concentration range encompassing the 50% scavenging concentration for DPPH-radicals from the regression line drawn in step 10.
Example of a Preliminary Experiment
Determination of the optimum concentration range
The optimum concentration range for gallic acid, a type of antioxidant, was determined as follows.
- The regression line for the concentration range of 1,000 – 0.001 μg/ml of gallic acid was plotted.
- The regression line indicated that the range between 10 and 100 μg/ml of gallic acid encompassed a 50% scavenging concentration.
The IC50 of gallic acid was calculated from the replotted regression line prepared from 5 to 100 μg/ml of gallic acid.
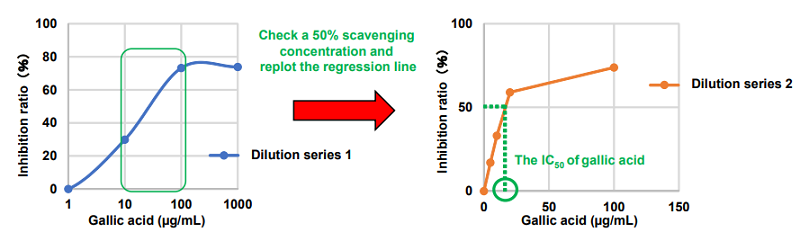
Figure 4. Example of an IC50 determination for gallic acid
Dilution series 1: 0.001, 0.01, 0.1, 1, 10, 100, and 1000 μg/ml
Dilution series 2: 5, 10, 20, and 100 μg/ml
Antioxidant Capacity Assay
- Table 2. Amount of solutions to be added
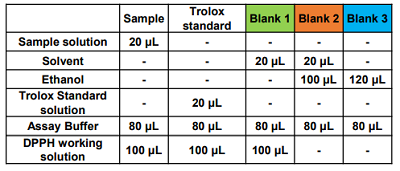
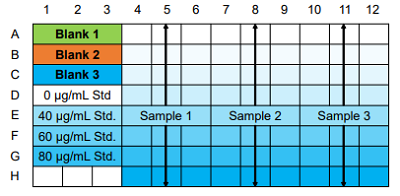
Figure 5. Example of a typical plate format
- Blank 1: coloring without antioxidant, Blank 2: sample solvent blank, Blank 3: ethanol blank
- If the sample is highly colored, prepare a sample blank for each concentration.
(1) Measurement of the DPPH-radical scavenging ratio of Trolox and unknown samples
- Please follow the order of this protocol because it is optimised for an antioxidant assay.
- Add 20 μl of 0, 40, 60, and 80 μg/ml of Trolox Standard solution to each well.
- Add 20 μl of the sample solution at four or more concentrations (using the optimum concentration range that was determined from a preliminary experiment), to each well.
- Add 20 μl of ethanol to the wells of Blank 3 and add 20 μl of the solvent that was used for sample dilution to the wells of Blank 1 and Blank 2.
- To avoid any concentration change because of volatilization, move to step 4 immediately.
- Add 80 μl of Assay Buffer to each well.
- Add 100 μl of ethanol to the wells of Blank 2 and Blank 3 and mix the wells by pipetting.
- Add 100 μl of DPPH working solution to the wells of Trolox, samples and Blank 1, and mix well by pipetting.
- Incubate the microplate at 25oC for 30 minutes in the dark.
- Measure the absorbance at 517 nm with a microplate reader.
- Calculate the inhibition ratio of the samples from the following equation:
Inhibition ratio of Trolox (%) = (AC - AR)/AC × 100 AC : Absorbance of 0 μg/ml Trolox Standard solution - Blank 3 AR : Absorbance of 40 to 80 μg/ml Trolox Standard solution - Blank 3 Inhibition ratio of sample (%) = (ACS - AS)/ACS × 100 ACS : Blank 1 - Blank 2 AS : Absorbance of samples - Blank 2 or sample blank (in case the sample is highly colored) - Plot the inhibition ratio (y) against the sample concentration (x) and draw a regression line (y=ax+b).
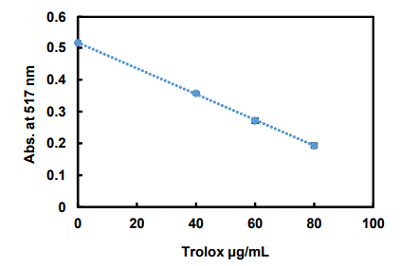
Figure 6. Absorbance changes of DPPH after Trolox treatment
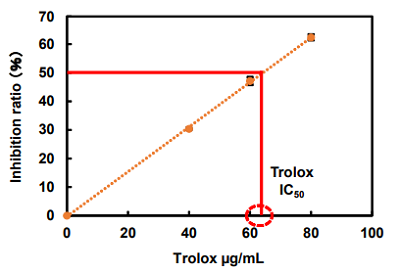
Figure 7. Inhibition ratio changes after Trolox treatment
(2) Calculation of the Trolox equivalent antioxidant capacity (TEAC)
- Calculate TEAC from the following equation:
TEAC = IC50 (Trolox)/ IC50(sample)
Reference
- T. Shimamura et al., Anal. Sci., 2014, 30, 717-721.
This product was commercialized under the advisory of Dr. Tomoko Shimamura (Faculty of Agriculture and Marine Science, Kochi University)
Frequently Asked Questions / Reference
D678: DPPH Antioxidant Assay Kit
Revised Apr., 28, 2023


 Hidden sections will not be printed.
Hidden sections will not be printed.