General Information
Autophagy is a degradation process of cytoplasmic dysfunctional proteins and organelles. In this process, an isolation membrane composed of a double membrane appears in cytosol, expands gradually, enfolds with the aggregated proteins and damaged organelles, and closes to form autophagosomes. The autophagosomes are fused with lysosomes to form autolysosomes, in which an acidic environment exists. The contents in autolysosomes are decomposed by digestive enzymes in lysosomes. Since this cellular function is said to be related to aging as well as neurodegenerative diseases such as Parkinson’s disease, a simple autophagy detection method is required.
DALGreen is a small fluorescent molecule. Because it has unique properties which emits fluorescence under hydrophobic and acidic conditions, DALGreen can detect the autolysosomes. DALGreen is cell permeable, has no requirement of transfection method, and enables live cell imaging with fluorescence microscopy and quantitative assay by flow cytometry.
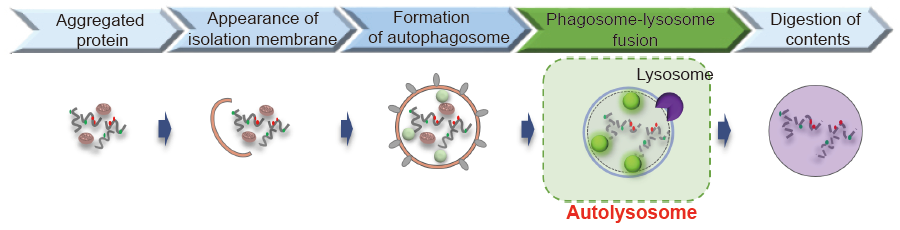
Fig. 1 The detection of autophagy with DALGreen
Contents
| DALGreen - Autophagy Detection | 20 nmol x 1 |
Storage Condition
Store at -20oC and protect from light.
Required Equipment and Materials
- Dimethyl sulfoxide (DMSO)
- Culture medium
- Hanks’ HEPES buffer or serum-free medium
- Micropipettes
Preparation of Solutions
Preparation of 1 mmol/l DALGreen DMSO stock solution
Add 20 μl of DMSO to a tube of DALGreen (20 nmol) and dissolve it with pipetting.
- Store the reconstituted DMSO solution at -20oC. The solution is stable at -20oC for 1 month.
Preparation of DALGreen working solution
Dilute the 1 mmol/l DALGreen DMSO stock solution with culture medium to prepare 0.1-1.0 μmol/l DALGreen working solution.
- Please optimize the final concentration of DALGreen depeneding on the cell lines.
General Protocol
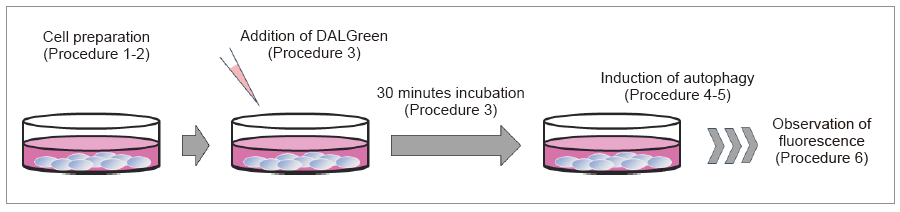
Autophagy detection
- Prepare cells on dish for assay.
- Discard the supernatant and wash the cells with culture medium.
- Add an appropriate volume of DALGreen working solution and then incubate at 37oC for 30 minutes.
- Discard the supernatant and wash the cells with culture medium twice.
- Add medium containing autophagy-inducing agent and incubate at 37oC.
- Please optimize the incubation time according to autophagy-inducing conditions.
- Observe fluorescence with a fluorescence microscope or flow cytometer.
Experimental Example
Observation on Confocal Fluorescence Microscopy
HeLa cells were seeded on μ-slide 8 well (Ibidi) and cultured at 37oC overnight in a 5% CO2 incubator. The cells were washed with culture medium and then incubated at 37oC for 30 minutes with 250 μl of 1 μmol/l DALGreen working solution. After the cells were washed with the culture medium twice, the culture medium or amino acid-free mediumthe (FUJIFILM Wako Pure Chemical, Ltd., Code: 048-33575) was added to the well. After 6 hours incubation, the cells were washed with Hanks’ HEPES buffer twice and then DALGreen was observed by confocal fluorescence microscopy.
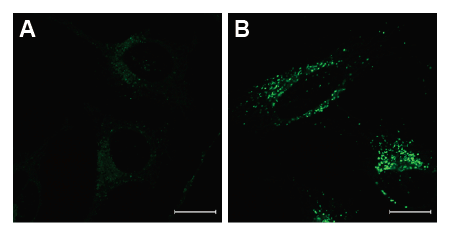
Fig. 2 Confocal microscopic images of various autophagy induced conditions.
After the addition of DALGreen, the cells were incubated with the culture medium (A) or the amino acid-free medium (B).
Fluorescence images were obtained using confocal microscopy at an excitation wavelength of 488 nm and a 500-563 nm emission filter. Scale bar: 20 μm.
Analysis by Flow Cytometry
HeLa cells were seeded on 24 well plate and cultured at 37oC overnight in a 5% CO2 incubator. The cells were washed with culture medium and then incubated at 37oC for 30 minutes with 1 μmol/l DALGreen working solution. After the cells were washed with the culture medium twice, the culture medium or amino acid-free medium was added to the well. After 20 hours incubation, the cells were washed with PBS, treated with trypsin and centrifuged. The pellets were suspended in Hanks’ HEPES buffer, and detected by flow cytometry.
-
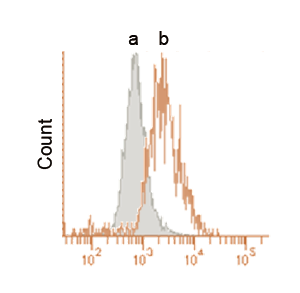
Fig. 3 Detection by flow cytometry.
-
After the addition of DALGreen, the cells were incubated with the culture medium (a) or the amino acid-free medium (b).
These data were obtained using flow cytometer at an excitation wavelength of 405 nm and a 485-535 nm emission filter.
Supplemental Information
Excitation and emission spectra of DALGreen
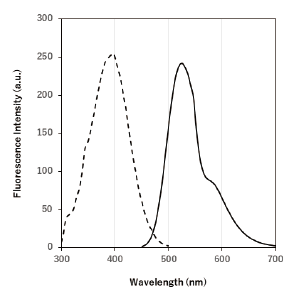 |
λex : 405 nm λem : 525 nm Ex:350 ~ 450 nm Em:500 ~ 560 nm |
Frequently Asked Questions / Reference
D675: DALGreen - Autophagy Detection
Revised Jul., 12, 2023


 Hidden sections will not be printed.
Hidden sections will not be printed.