General Information
Cytotoxicity LDH Assay Kit-WST is a kit for determination of cytotoxicity by measuring a lactate dehydrogenase (LDH) activity released from damaged cells. LDH is a stable cytoplasmic enzyme presented in all types of cells and released into the cell culture medium through damaged plasma membrane. Cytotoxicity LDH Assay Kit-WST can be used to measure the released LDH according to the following scheme. LDH catalyzes dehydrogenation of lactate to pyruvate thereby reducing NAD to NADH. NADH reduces a water-soluble tetrazolium salt (WST) in the presence of an electron mediator to produce an orange formazan dye. The amount of the formazan dye thus formed is proportional to that of released LDH into the medium, which is an indication of cytotoxicity.
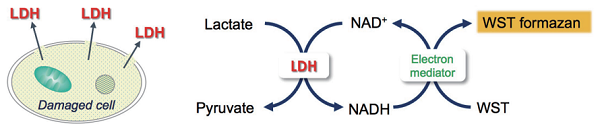
Principle of cytotoxicity measurement
Since Cytotoxicity LDH Assay Kit-WST neither reflects the activity of living cells nor is harmful to cells, cytotoxicity can be measured with the living cells (homogeneous assay). In addition, non-homogeneous assay that is performed by using the cell culture supernatant is also possible. Unlike competitive products, the reconstituted Working Solution is stable under refrigerated condition and the Working Solution can be used for long periods as ready-to use solution after the preparation.
-
Homogeneous assay
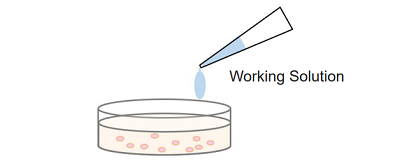
Simple procedure: homogeneous assay only requires adding the Working Solution to each well in the presence of live and dead cells, there is no need to transfer cell culture supernatant to a new microplate for measurement.
-
Non-homogeneous assay
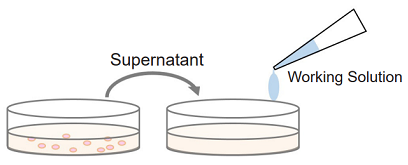
Multi-measuring use: since non-homogeneous assay uses the culture supernatant from each well for the measurement of LDH activity, the sample cells in each well can be applied to some other experiments such as cell viability assay (WST-8 and MTT) and cell staining (nuclear staining, immunostaining).
Kit Contents
| 100 tests | 500 tests | 2000 tests | |
| Dye Mixture | × 1 | × 1 | × 4 |
| Assay Buffer | 11 ml × 1 | 55 ml × 1 | 55 ml × 4 |
| Lysis Buffer | 1.1 ml × 1 | 5.5 ml × 1 | 5.5 ml × 4 |
| Stop Solution | 5.5 ml × 1 | 27.5 ml × 1 | 27.5 ml × 4 |
Storage Condition
Store at 0-5°C
Required Equipment and Materials
- CO2 incubator
- 20, 100-200 μl multichannel pipettes
- Microplate reader (490 nm filter)
- 96-well tissue culture plate*1
- 96-well optically clear plate (flat-bottomed)*2
- For suspension cells in non-homogeneous assay: round or V-bottomed plate.
- For non-homogeneous assay.
Precaution
- This kit contains a glass bottle with an aluminum cap. Use protective gloves and be cautious in handling.
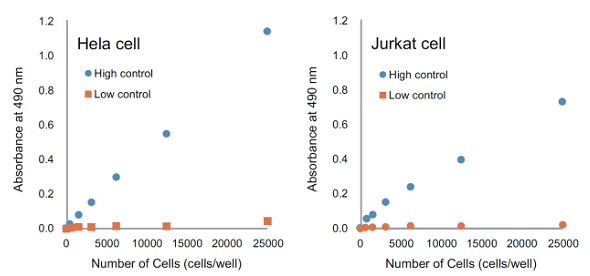
- The amount of LDH is dependent on the cell types. We recommend carrying out a preliminary experiment to optimize the cell concentration.
Optimization of Cell Number
The amount of intracellular LDH is different in each type of cells. Therefore, it is required to optimize the number of cells to obtain reliable results before performing your cytotoxicity assay.
| 1. Optimlization of cell number |
| If you are using the product for the first time, or if you are measuring different cell types, please optimize the number of cells. Measure each absorbance of high control, low control and background control, and then determine the number of cells for cytotoxicity assay.
Procedure
|
| 2. Cytotoxicity Assay |
|
Perform the cytotoxicity assay using the optimized number of cells per well.
|
Preparation of Reagent
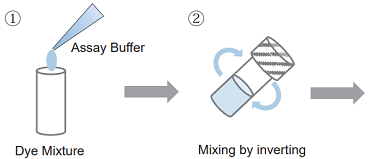
- Add appropriate volume of Assay Buffer to a Dye Mixture vial and close the cap.
100 tests : 1 ml of Assay Buffer to a Dye Mixture vial. 500 tests /2000 tests : 5 ml of Assay Buffer to a Dye Mixture vial. - Dissolve the contents completely.
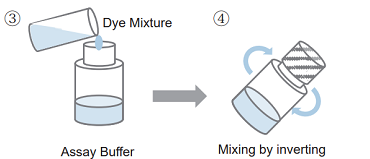
- Transfer all of the solution of step 2 to Assay Buffer bottle.
- Close the cap and mix it well.
- After preparing Working Solution, please store it at 0-5°C and protect from light. Under this condition, it is stable for six months.
Optimization of Cell Number - Homogeneous assay -
Optimization of cell number -Homogeneous assay-
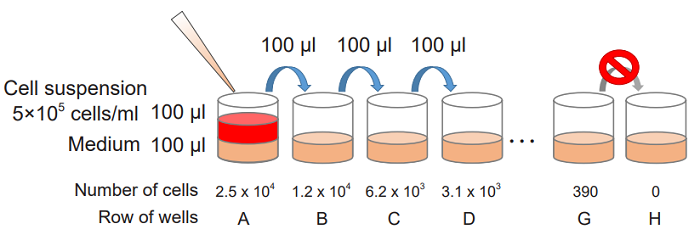
- Collect cells and wash them with the medium. Prepare cell suspension at 5 x 105 cells/ml with the medium.
- Add 100 μl of the medium to each well of a flat-bottom 96-well culture plate.
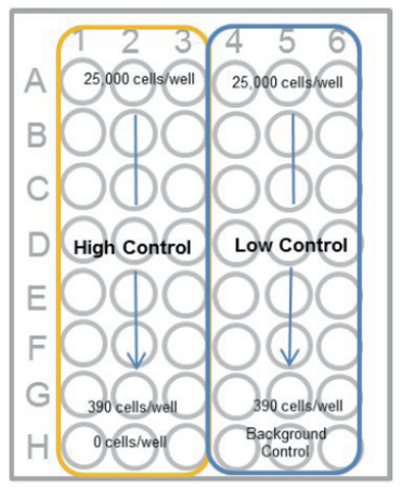
- Prepare 2-fold serial dilution of each well in triplicate set of wells for the high control, low-control and background control (medium only).
-
Serial dilution procedure
Add 100 μl of the cell suspension (5 x 105 cells/ml) to the first well [ A ] and mix by pipetting.
This well contains the maximum number of cells (2.5 x 104 cells/well).
Transfer 100 μl from the first well to the next well [ B ], and mix by pipetting.
Repeat this procedure.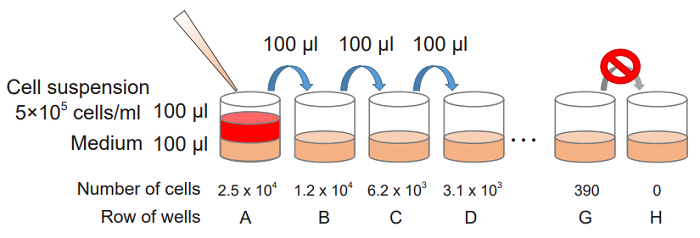
-
- Incubate the plate at 37°C for an appropriate time in a CO2 incubator.
- The incubation time should be determined according to how long cells are exposed to test substance.
- Add 10 μl of the Lysis Buffer to each well of the high control.
- The amount of Lysis Buffer to be added is small, therefore the tip of the pipette should be touched to the wall. If the reagent remains on the wall, please tap the plate gently and mix with the medium.
- Incubate the plate at 37°C for 30 minutes in a CO2 incubator.
- Add 100 μl of the Working Solution to each well. Protect the plate from light and incubate it at the room temperature for 30 minutes.
- Add 50 μl of the Stop Solution to each well.
- Measure the absorbance at 490 nm by a microplate reader.
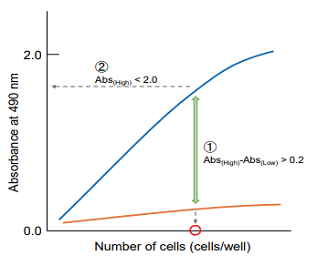
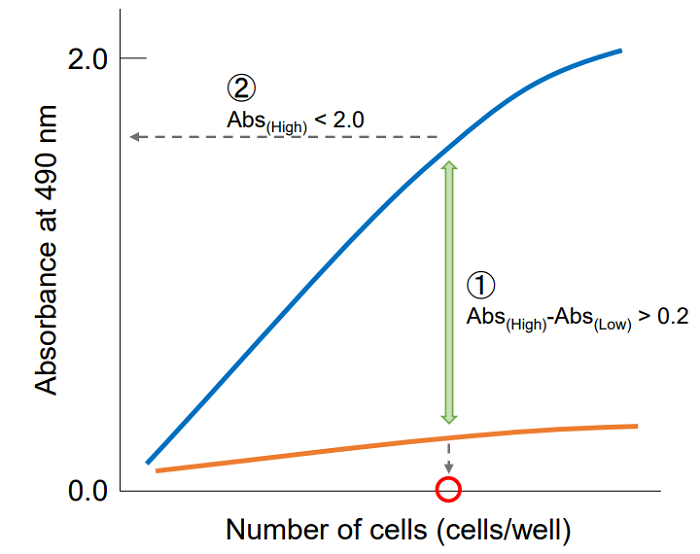
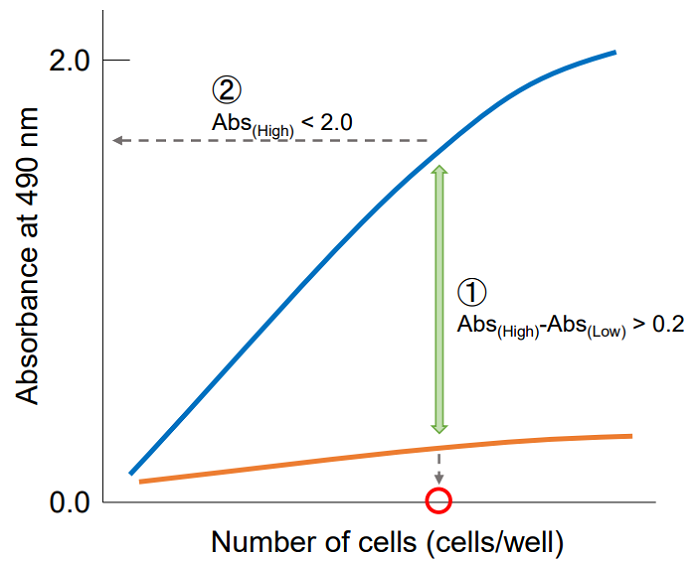
Plot the data by setting cell concentration for the x-axis and absorbance for the y-axis. Then, find the optimum cell number to meet the following condition.
① The difference in the absorbance between high control and low control is at least 0.2.
② The absorbance is lower than 2.0 and positioned on the linear point of plotted curve.
Cytotoxicity Assay - Homogeneous assay -
Cotoxicity Assay - Homogeneous assay -
- Add 50 μl of cell suspension to each well of a flat-bottom 96-well culture plate (see table below).
- Prepare the cell suspension so that the number of cells contained in 50 μl of the cell suspension is the same as the number of cells determined in the preliminary experiment.
- For adherent cells, please incubate the culture plate overnight to attach the cells to the plate. After incubation, replace the medium with 50 μl of the fresh medium and proceed to step 2. For suspension cells, skip this procedure.
- Add medium to each well for the high-control, low-control and background control (see table below).
- Add 50 μl of medium containing test substance that adjusted to the desired concentration.
- Test substance should be prepared to the target concentration with the medium before use it.
Table. Overview of the controls (Homogeneous Assay) Test substance High control* Low control Bacground control Medium - 50 μl 50 μl 100 μl Cell suspentions 50 μl 50 μl 50 μl - Test substance in culture medium 50 μl - - - Lysis Buffer - 10 μl - - - The volume of high control increases by 10% against other controls. However, a volume adjustment is not necessary because this volume difference does not affect the experimental results.
Test substance : LDH activity released from the cell treated with the test substance High control : Total LDH activity in the cell (= Maximum LDH release) Low control : LDH activity released from the untreated cell (= Spontaneous LDH release) Bacground control : LDH activity in the culture medium - Incubate the plate at 37°C for an appropriate time in a CO2 incubator.
- Please determine the appropriate exposure time of cells to the test substance according to the experimental condition.
- Please determine the appropriate exposure time of cells to the test substance according to the experimental condition.
- Add 10 μl of the Lysis Buffer to each well of the high control.
- The amount of Lysis Buffer to be added is small, therefore the tip of the pipette should be touched to the wall. If the reagent remains on the wall, please tap the plate gently and mix with the medium.
- Incubate the plate at 37°C for 30 minutes in a CO2 incubator.
- Add 100 μl of the Working Solution to each well. Protect the plate from light and incubate it at the room temperature for 30 minutes.
- Add 50 μl of the Stop Solution to each well.
- Measure the absorbance at 490 nm by a microplate reader.
【Caluculation of cytotoxicity】
- Calculate the average absorbance from each triplicate set of wells and subtract the background control value from each absorbance.
- Determine the percent of cytotoxicity by a following equation.

Optimization of Cell Number - Non-homogeneous assay -
Optimization of cell number - Non-homogeneous assay -
- Collect cells and wash them with the medium. Prepare cell suspension at 5 x 105 cells/ml with the medium.
- Add 100 μl of the medium to each well of a flat-bottom 96-well culture plate.
- Prepare 2-fold serial dilution of each well in triplicate set of wells for the high control, low-control and background control (medium only).
〈Serial dilution procedure〉
Add 100 μl of the cell suspension (5 x 105 cells/ml) to the first well [ A ] and mix by pipetting.
This well contains the maximum number of cells (2.5 x 104 cells/well).
Transfer 100 μl from the first well to the next well [ B ], and mix by pipetting.
Repeat this procedure.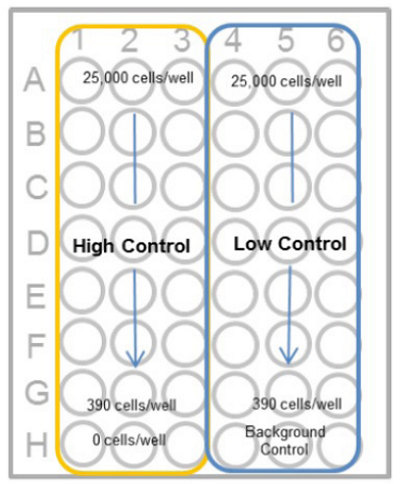
- Add 100 μl of the medium to each well.
- Incubate the plate at 37°C for an appropriate time in a CO2 incubator.
- The incubation time should be determined according to how long cells are exposed to test substance.
- Add 20 μl of the Lysis Buffer to each well of the high control.
- The amount of Lysis Buffer to be added is small, therefore the tip of the pipette should be touched to the wall. If the reagent remains on the wall, please tap the plate gently and mix with the medium.
- Incubate the plate at 37°C for 30 minutes in a CO2 incubator.
- For suspension cells, centrifuge the plate at 250 x g for 2 minutes to deposit the cells.
- For adherent cells, you can skip this step.
- Transfer 100 μl of the supernatant from each well to an optically clear flat-bottom 96-well plate.
- Add 100 μl of the Working Solution to each well. Protect the plate from light and incubate it at the room temperature for 30 minutes.
- Add 50 μl of the Stop Solution to each well.
- Measure the absorbance at 490 nm by a microplate reader.
Plot the data by setting cell concentration for the x-axis and absorbance for the y-axis. Then, find the optimum cell number to meet the following condition.
① The difference in the absorbance between high control and low control is at least 0.2.
② The absorbance is lower than 2.0 and positioned on the linear point of plotted curve.
Cytotoxicity Assay - Non-homogeneous assay -
Cotoxicity Assay -Non-homogeneous assay-
- Add 100 μl of cell suspension to each well of a flat-bottom 96-well culture plate (see table below).
- Prepare the cell suspension so that the number of cells contained in 100 μl of the cell suspension is the same as the number of cells determined in the preliminary experiment.
- For adherent cells, please incubate the culture plate overnight to attach the cells to the plate. After incubation, replace the medium with 100 μl of the fresh medium and proceed to step 2. For suspension cells, skip this procedure.
- Add medium to each well for the high-control, low-control and background control (see table below).
- Add 100 μl of medium containing test substance that adjusted to the desired concentration.
- Test substance should be prepared to the target concentration with the medium before use it.
Table. Overview of the controls (Non-homogeneous Assay) Test substance High control Low control Bacground control Medium 20 μl 100 μl 120 μl 220 μl Cell suspentions 100 μl 100 μl 100 μl - Test substance in culture medium 100 μl - - - Lysis Buffer - 20 μl - - Test substance : LDH activity released from the cell treated with the test substance High control : Total LDH activity in the cell (= Maximum LDH release) Low control : LDH activity released from the untreated cell (= Spontaneous LDH release) Bacground control : LDH activity in the culture medium - Incubate the plate at 37°C for an appropriate time in a CO2 incubator.
- Please determine the appropriate exposure time of cells to the test substance according to the experimental condition.
- Add 20 μl of the Lysis Buffer to each well of the high control.
- The amount of Lysis Buffer to be added is small, therefore the tip of the pipette should be touched to the wall. If the reagent remains on the wall, please tap the plate gently and mix with the medium.
- Incubate the plate at 37°C for 30 minutes in a CO2 incubator.
- For suspension cells, centrifuge the plate at 250 x g for 2 minutes to deposit the cells.
- For adherent cells, you can skip this step.
- Transfer 100 μl of the supernatant from each well to an optically clear flat-bottom 96-well plate.
- Add 100 μl of the Working Solution to each well. Protect the plate from light and incubate it at the room temperature for 30 minutes.
- Add 50 μl of the Stop Solution to each well.
- Measure the absorbance at 490 nm by a microplate reader.
【Caluculation of cytotoxicity】
- Calculate the average absorbance from each triplicate set of wells and subtract the background control value from each absorbance.
- Determine the percent of cytotoxicity by a following equation.

Troubleshooting
| Problem | Possible cause | Recommendation |
| Large variation of absorbance | Bubbles are in the medium. | Please break bubbles by using a needle.If you have a centrifuge for the 96-well plate, please centrifuge it for a minute with 1,000 x g. |
| Test substance concentration changes by the evaporation of the medium. | Evaporation easily occurs in the outermost wells of the microplate. Therefore, if the plate is incubated for a long time, please add the medium in the outermost wells and do not use them for an assay. | |
| Reagents are not mixed well. | Please be noted that the amount of the added Lysis Buffer is less than other reagents, and hence it can affect the High Control values. Please mix each reagent and the medium by a plate mixer or tap the surface of the plate with fingers gently when Lysis Buffer, Working Solution, and Stop Solution are added. Also, do not tap the plate too hard so that the medium inside the well would not leak. | |
| Reagent volume are not accurate. | Calibrate the pipett. | |
| Reaction time varies among wells by using a single channel pipette. | Multichannel pipettes are recommended to add Working Solution and Stop Solution. | |
| High background values | Medium contains high concentration of LDH. | Use the serum-free medium or the medium contains the serum less than 5%. |
| Test substance or the medium has reducing activity. | Some reducing compounds such as ascorbic acid react directly with WST dye. Use medium such as DMEM, RPMI, F-12 do not contain reducing compounds. | |
| Low absorbance values | Test substance or medium inhibit LDH activity or the colorimetric reaction. | Refer to "Regarding interfering substance" below. |
- Reducing agents
Since some reducing agents such as ascorbic acid cause a high background, determine if test substance increases the O.D. value of the medium containing the working solution in the absence of cells. This background can be subtracted as the test substance background*.
*In this case, the O.D. values of the test substance background are required. Test substance: test substance + medium + working solution.
- LDH included serum in the medium
LDH contained in the medium increases the O.D. value of each well. The background from the LDH can be subtracted from the O.D. value of each sample. If the background values are too high, use serum-free medium or reduce the concentration of serum less than 5%.
- Inhibitors of LDH activity
For example, the medium containing pyruvic acid in high concentration inhibits the colorimetric reaction between LDH and WST. In case the O.D. value of high control is low (close to that of low control in the preliminary experiment), determine if pyruvic acid is contained in the medium.
Frequently Asked Questions / Reference
CK12: Cytotoxicity LDH Assay Kit-WST
Revised Sep., 01, 2023


 Hidden sections will not be printed.
Hidden sections will not be printed.