Introduction
Cell Counting Kit-F(CCK-F) offers simplified and highly sensitive cell proliferation and cytotoxicity assay method by employing Calcein-AM(3’,6’-Di(O-acetyl)-4’,5’-bis[N,N-bis(carboxymethyl)aminomethyl]fluorescein, tetraacetoxymethyl ester) that produces a highly sensitive fluorescent dye(λex=490 nm, λem=515 nm)upon enzymatic hydrolysis by esterases in living cells. The amount of the fluorescent dye, calcein, produced by hydrolysis by esterases in cells is directly proportional to the number of viable cells in a culture medium (Fig. 1).
Furthermore, CCK-F assay does not require any radioisotopes(such as in [3H]-thymidine incorporation assay)nor solubilization procedure(such as in MTT assay),and no special skills are necessary for use. Therefore, it allows you to obtain highly reprodutive and accurate results.
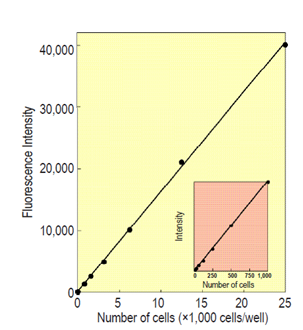
| Cell line: HL60 Incubation time: 30 min Detection: λex=485 nm, λem=535 nm |
Fig.1 A relationship between fluorescence intensity and number
Kit Contents
| Calcein-AM DMSO solution | 110 μl x 1 |
Required equipment and materials
- Fluorescence microplate reader (excitation filter: 480-500 nm, emission filter: 500-535 nm)
- 96-well microplate for fluorescent measurements (black plate or white plate)
Storage
Store at -20°C. This product is stable for six months at -20°C before opening.
If the solution remains, close the bottle cap tightly and store in a freezer at -20°C.
Working solution
Required amount of Calcein-AM DMSO solution is diluted 50 times with PBS(-). Since Calcein-AM is not stable in PBS(-), prepare the working solution immediately before use.
Cytotoxicity assay procedure
- Dispense 100 μl of cell suspension (5,000 cells/well) onto a 96-well microtiter plate.
- Preincubate the plate for 24 hours in an incubator (humidified atmosphere, e.g.,at 37°C, 5% CO2).
- Add 10 μl of various concentrations of the test substance into the culture medium of the plate.
- Incubate cell cultures for 48 hours in the incubator.
- Replace the medium with 100 μl of PBS(-). Use a centrituge for microplate in the case of nonadherent type cells before removing the culture medium.
- Add 10 μl of the working solution and incubate cell cultures for 15-30 min in the incubator.
- Measure the fluorescent(λex=490 nm, λem=515 nm) using a fluorescence microplate reader.
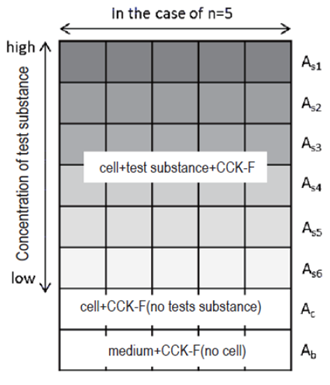
Fig. 2 An example of sample arrangement
Determination of IC50
Cell viability is calculated using the following equation. IC50, concentration killing 50% of the cells, is determined from plot of viability versus concentration of the test substance.
Viability (%) = [(As-Ab)/(Ac-Ab)] x 100
As: Fluorescence intensity of sample (cell + test substance + CCK-F)
Ac: Fluorescence intensity of control (cell + CCK-F, no test substance)
Ab: Fluorescence intensity of blank (medium + CCK-F, no cell)
Example of toxicological test using CCK-F is shown in Fig. 3.
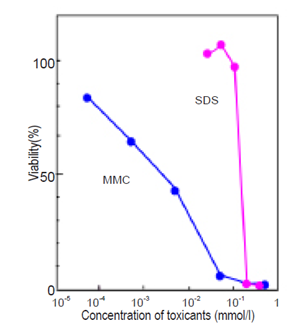
| Cell line: HL60 Toxicants: Mitomycin C (MMC), Sodium dodecylsulfate(SDS) Condition: 37°C, 5% CO2, 48 hrs Incubation time: 30 min Detection: λex=485 nm, λem=535 nm |
Fig.3 Toxicological test for MMC and SDS by CCK-F.
Caution
- Phenol red and serum in a culture medium interfere with the fluorescence measurement. Replace a culture medium with PBS(-) or phenol red and serum free medium prior to adding the working solution.
- Dilute the test substance with non-toxic solution, such as culture medium or PBS(-) or saline. And use same solution for control and blank wells instead of sample.
- If a 24-well or 6-well plate is used for this assay, calculate the number of cells per well accordingly, and adjust the volume of the working solution in a well to 10% of the total volume.
Frequently Asked Questions / Reference
CK06: Cell Counting Kit-F
Revised Apr., 12, 2024


 Hidden sections will not be printed.
Hidden sections will not be printed.