General Information
The transition metal copper is a trace element in the human body that enhances enzymatic activity related to vital functions including respiration and metabolism. For example, copper helps transport iron for hemoglobin synthesis by promoting iron metabolism, and is bound at the catalytic site of the enzyme superoxide dismutase that removes reactive oxygen species. Thus, copper plays a vital role in the maintenance of life.
Cuprotosis is a newly discovered copper-dependent type of cell death1). It has been demonstrated that cuproptosis differs from apoptosis and ferroptosis.
CuprosGreen is a fluorescent probe that selectively reacts with intracellular Cu(I) ions and emits fluorescence. Its cell membrane permeability enables live cell imaging of Cu(I) ions.
Content
CuprosGreen 20 μl×1
Storage Condition
Store at -20℃
Required Equipment and Materials
- Fluorescence microscope, or fluorescence plate reader
- Incubator (37℃)
- Micropipettes (100–1000 μl, 20–200 μl, 0.5–10 μl)
- Microtubes
- Medium or Hanks' balanced salt solution (HBSS)
- Dimethyl sulfoxide (DMSO)
Precaution
- Allow the CuprosGreen to equilibrate to room temperature before use.
- Centrifuge the tube briefly before opening the cap because the content may be on the tube wall or the inside of the cap.
- Please refer to Table 1 for fluorescence wavelengths for each application.
Table 1. Recommended filter settings of the CuprosGreen
| Applications | Fluorescence microscope | Fluorescence plate reader |
| Measurement wavelength |
・Confocal microscope Ex/Em: 488/500–600 nm ・Fluorescence microscope GFP filter |
Excitation: 430–480 nm Emission: 510–560 nm |
Preparation of Solutions
Preparation of 5 μmol/l CuprosGreen working solution
- Take a necessary amount of the CuprosGreen with a micropipette and transfer it to a microtube.
- Add one and a half times amount of DMSO to a tube containing CuprosGreen, then mix by pipetting.
- Add five-hundred-fold of serum free medium to the tube, then mix by pipetting and voltex.
Note: Use a serum-free medium to prepare the working solution for optimal performance of CuprosGreen.
Note: The working solution cannot be stored and must be prepared freshly on the day of use.
Note: Refer to Table 2 for information for the preparation of working solution by vessel type.
e.g. : Preparation of 1 ml of CuprosGreen working solution
- Add 2 μl of CuprosGreen to a new tube.
- Add 3 μl of DMSO to the tube, then mix by pipetting.
- Add 1000 μl of serum free medium to the tube, then mix by pipetting and voltex.
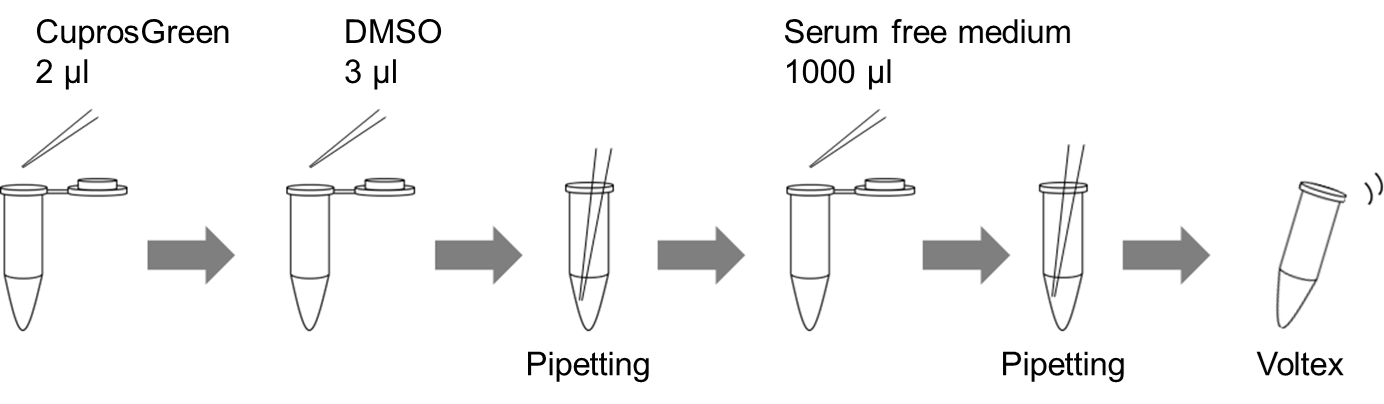
Figure 1. Preparation of 1 ml of CuprosGreen working solution
Table 2. Required amount of the working solution by vessel type
| Vessel | 35-mm dish | ibidi 8-well plate | 96-well black plate (clear bottom) |
| Appropriate amount | 2 ml | 200 μl/well | 100 μl/well |
General protocol
- Seed cells on a dish. In an incubator, culture the cells overnight at 37°C with 5% CO2.
- Discard the supernatant and wash the cells once with the growth medium.
- Discard the supernatant, add a medium containing stimulation agents, and incubate the cells as in step 1 for an appropriate time.
- Discard the supernatant and wash the cells twice with serum-free medium.
- Discard the supernatant and add an appropriate volume of CuprosGreen working solution to the cells.
- Incubate the cells as in step 1 for 3 h.
- Observe fluorescence signals using each detector.
Note: Do not wash the cells after step 6.
Usage example
Detection of the intracellular Cu (Ⅰ) in HeLa cells treated with CuCl2
- HeLa cells in MEM (supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin) were seeded into vessels and incubated overnight at 37°C in 95% air/5% CO2.
- After removing the culture medium, the cells were washed once with the culture medium.
- After removing the supernatant, MEM (supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin) containing CuCl2 (200 μmol/l) was added to the cells.
- The cells were incubated for 16 h as in step 1.
- The supernatant was removed, and the cells were washed twice with PBS containing EDTA-2Na (200 μmol/l).
- After removing the supernatant, 5 μmol/l CuprosGreen working solution was added and the cells were incubated for 3 h as in step 1.
- Fluorescence signals were measured using a plate reader, and imaging was performed by fluorescence microscopy.
- Number of seeded HeLa cells in each vessel
96-well black plate, 1×104 cells/well; ibidi 8-well plate, 3×105 cells/well.
- Amount of each solution added to each vessel
96-well black plate, 100 μl/well; ibidi 8-well plate, 200 μl/well.
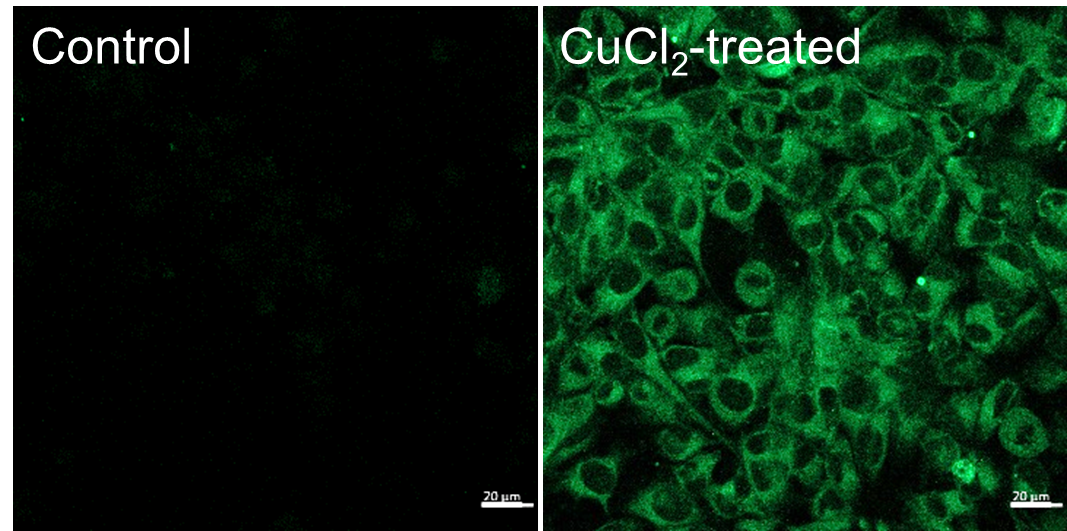
Ex/Em: 488/500–600 nm, Scale bar: 20 μm
Figure 1. Fluorescence signals from HeLa cells detected with a confocal laser microscope
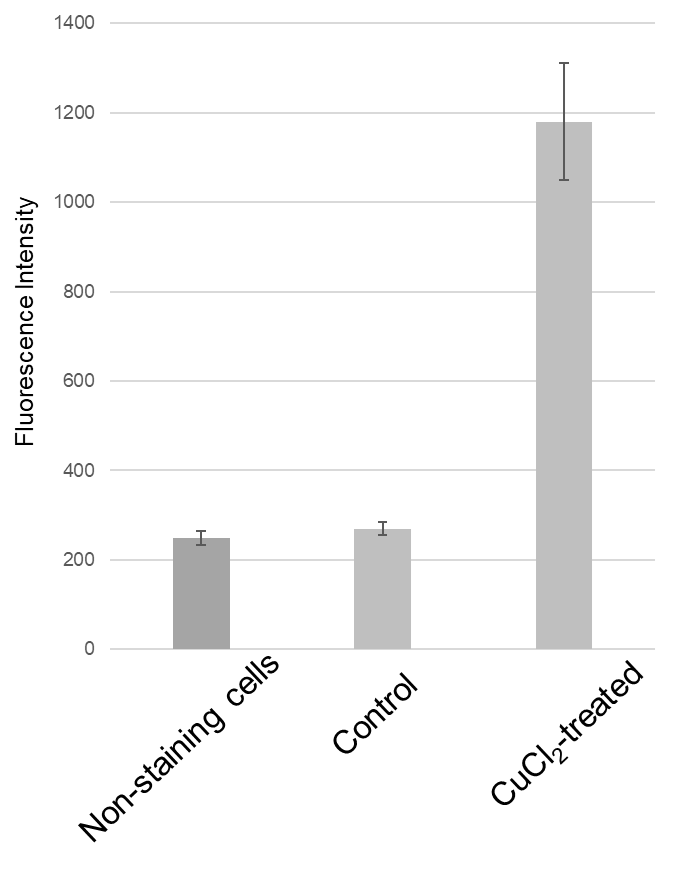
Ex/Em: 470/510 nm
Figure 2. Fluorescence signals from HeLa cells detected with a plate reader
Supplemental Infomation
Metal ion selectivity of CuprosGreen
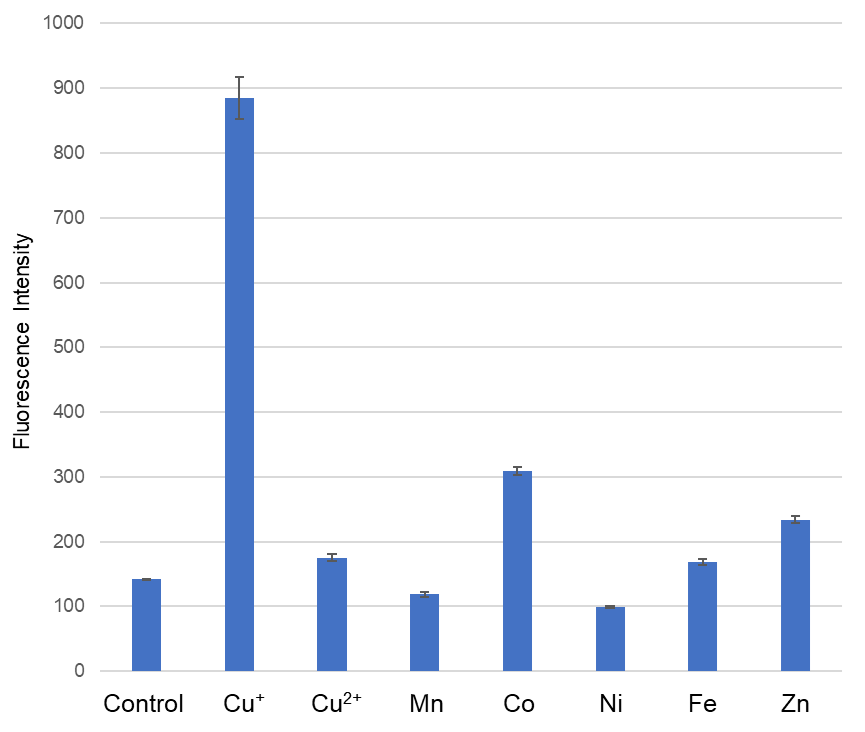
Ex/Em: 470/510 nm,
Buffer: 50 mmol/l HEPES (pH 7.2), CuprosGreen: 1 μmol/l, Metal: 20 μmol/l, incubation time: 1 h
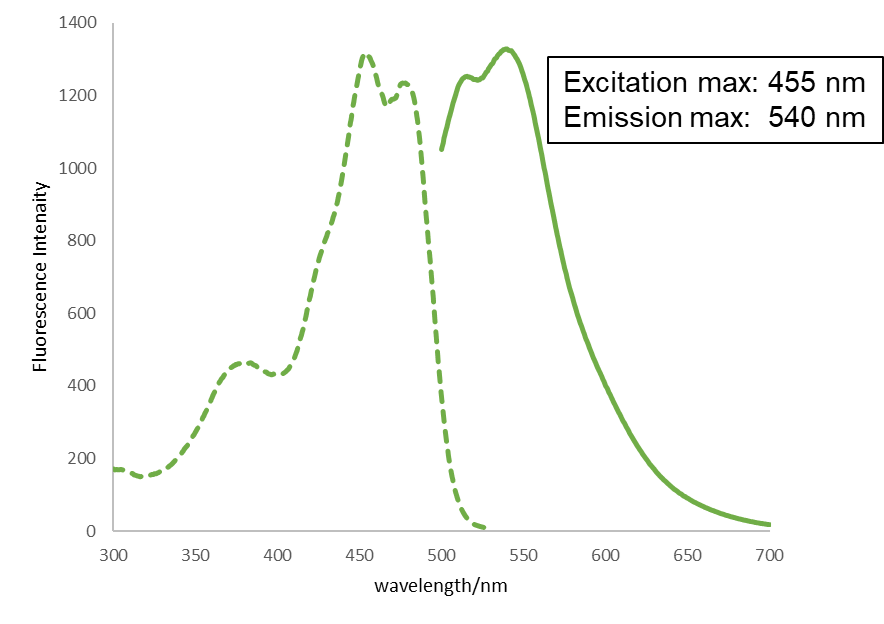
Excitation and emission spectra of CuprosGreen
Reference
- Tsvetkov et al., Science, 2022, 375, 1254–1261
Frequently Asked Questions / Reference
C557: CuprosGreen
Revised Oct., 25, 2024


 Hidden sections will not be printed.
Hidden sections will not be printed.

