General Information
Flow cytometers are often used in immunology research to analyze immune cells. Immune cells possess characteristic surface antigens, and by performing immunostaining using fluorescence-labeled antibodies specific to these surface markers, analysis by flow cytometer becomes possible. In this case, dead cells in the sample can cause nonspecific binding of antibodies in flow cytometry, leading to a decrease in the reliability of the analysis. To increase the reliability of the data, it is necessary to stain dead cells and separate them from live cells.
Propidium Iodide (PI) is used for this purpose. However, PI leaks out of dead cells due to cell immobilization and membrane permeabilization, and staining of immobilized live cells results in false-positive data.
Dead Cell Makeup Dyes cannot penetrate live cell membranes but can permeate the damaged membranes of dead cells and form covalent bonds with intracellular proteins. Therefore, Dead Cell Makeup Dyes do not leak from the cells even after compromised membrane integrity by cell fixation and membrane permeabilization procedures (Figure 1). In addition, the resulting fluorescence intensity between live and dead cells has significant differences (Figure 2), allowing easy to distinguish dead and live cells in flow cytometric analysis.
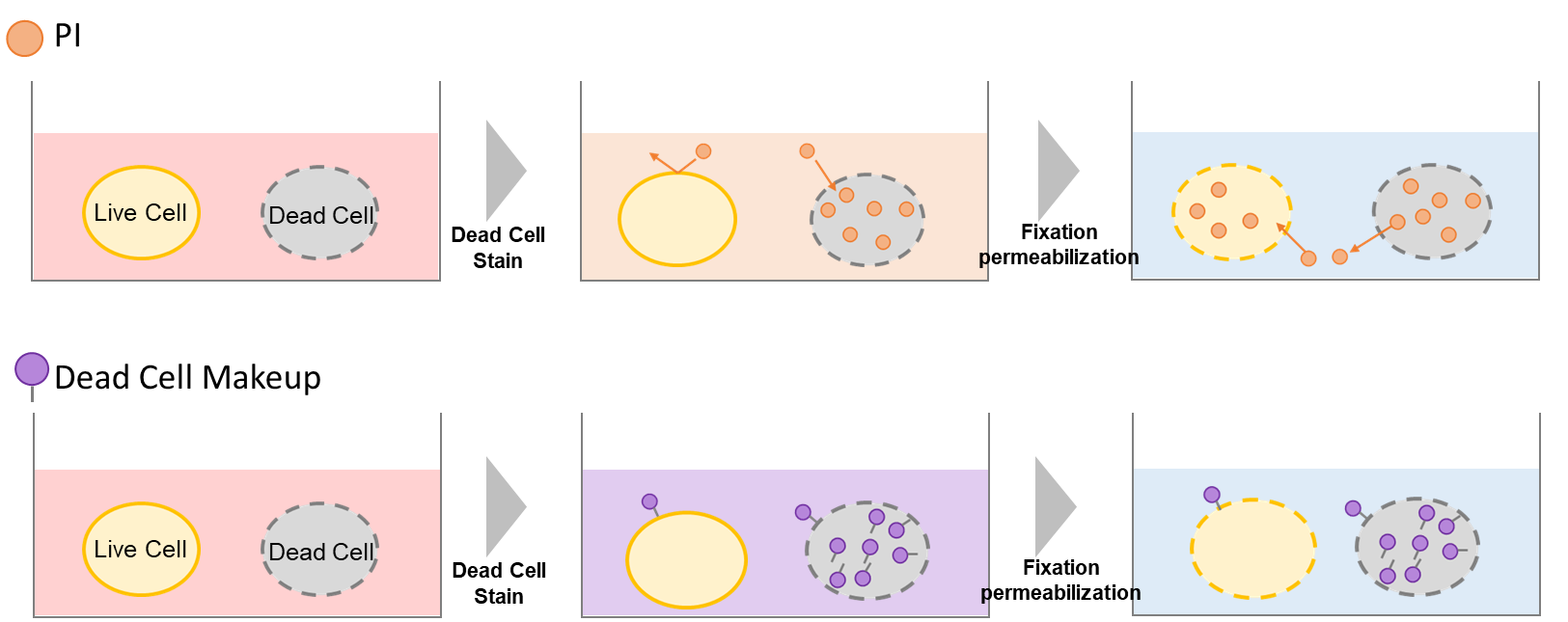
Figure1. Principle of Dead Cell Makeup Dyes
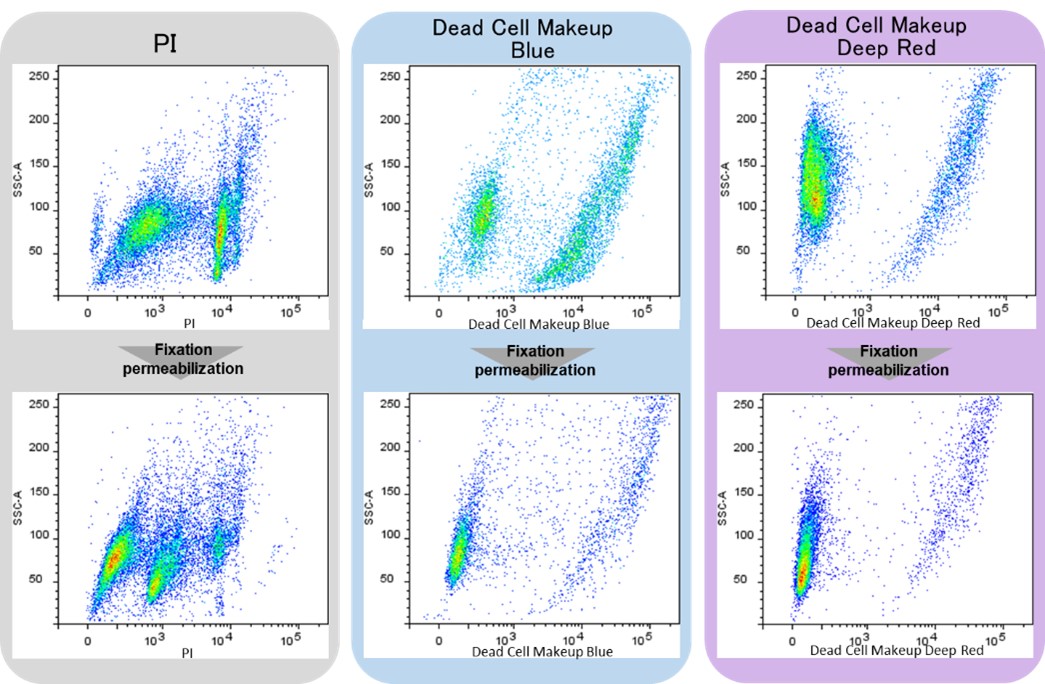
Figure 2. Flow cytometic analysis of PI and Dead Cell Makeup Dyes
Contents
| C555 : | Dead Cell Makeup Blue | x1 |
| C556 : | Dead Cell Makeup Deep Red | x1 |
- Dead Cell Makeup Blue in the tube may be barely visible due to the colorless form. Please handle it carefully.
Storage Condition
| C555 : | Store at 0–5°C and protected from light. |
| C556 : | Store at -20°C and protected from light. |
Required Equipment and Materials
- Flow cytometer
- Cell strainer for flow cytometer
- Centrifuge
- Microcentrifuge tubes
- Micropipettes
- Dimethylsulfoxide (DMSO)
- Phosphate-buffered saline (PBS)
Preparation of Solutions
Preparation of Dead Cell Makeup Dye DMSO stock solution
Add 50 µl of DMSO to Dead Cell Makeup Dye and dissolve by vortexing and pipetting.
- Store the Probe stock solution at -20°C, protect from light. The probe stock solution can be stored for 6 months.
- Please use fresh DMSO to prepare a stock solution for long-term storage of more than 6 months.
Aliquot the DMSO solution in an appropriate volume and store at -20°C as necessary.
Preparation of Dead Cell Makeup Dye working solution
Dilute Dead Cell Makeup Dye DMSO stock Solution 1,000 times with PBS to prepare Dead Cell Makeup Dye working Solution.
- Please use up the Dead Cell Makeup working solution within that day.
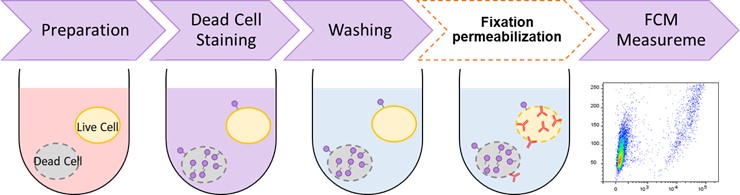
General Protocol
- Prepare a cell suspension (1–10 x 106 cells/ml).
- For adherent cells, harvest the cells using a cell scraper or trypsinization and prepare a cell suspension.
- Add 1 ml of cell suspension in a 1.5 ml microcentrifuge tube and centrifuge at 300 x g for 5 min.
- Discard the supernatant, add 500 μl of Dead Cell Makeup Dye working solution to the microcentrifuge tube, and suspend by pipetting.
- Incubate at room temperature for 15 min and protect from light, centrifuge at 300 x g for 5 min.
- Discard the supernatant, add 300 μl of PBS to the microcentrifuge tube, and suspend by pipetting.
- Centrifuge at 300 x g for 5 min.
- Discard the supernatant, add 300 μl of PBS to the microcentrifuge tube, and suspend by pipetting.
- Pass through a cell strainer for a flow cytometer.
- Analyze samples using a flow cytometer.
- Performing cell fixation and membrane permeabilization after step 7.
|
Code |
Product name | Excitation | Emission |
| C555 | Dead Cell Makeup Blue | 405 nm |
450 / 50 nm Ex.) Pacific Blue・Alexa Fluor 405・Brilliand Violet 421 |
| C556 | Dead Cell Makeup Deep Red | 640 nm |
670 / 30 nm Ex.) APC. Alexa Fluor 647. Cy5 |
Experimental Example
PD-1 detection after exhaustion induction of MOLT-4 cell
- MOLT-4 cell suspension was prepared (5 x 106 cells/ml).
- The supernatant was discarded, RPMI containing 500 ng/ml Ionomycin and 50 ng/ml PMA (Phorbol 12-myristate 13-acetate) was added, and the cells were incubated for 48 h in a 37oC incubator equilibrated with 95 % air and 5% CO2.
- One ml of the cell suspension was transferred to a 1.5 ml microcentrifuge tube and centrifuged at 300 x g for 5 min.
- The supernatant was discarded, and 500 μl of Dead Cell Makeup Dye working solution was added to the microcentrifuge tube and suspended by pipetting.
- The microcentrifuge tube was incubated at room temperature for 15 min, protected from light, and centrifuged at 300 x g for 5 min.
- The supernatant was discarded, and 300 µl of PBS was added to the microcentrifuge tube, and suspended by pipetting.
- The microcentrifuge tube was centrifuged at 300 x g for 5 min.
- The supernatant was discarded, and 100 μl of 4% PFA (PBS) was added to the microcentrifuge tube and suspended by pipetting.
- The microcentrifuge tube was incubated at room temperature for 30 min and centrifuged at 1,000 x g for 5 min.
- The supernatant was discarded, and 100 μl of 0.1% Triton X-100 (PBS) was added to the microcentrifuge tube and suspended by pipetting.
- The microcentrifuge tube was incubated at room temperature for 30 min and centrifuged at 1,000 x g for 5 min.
- The supernatant was discarded, and 300 μl of 10% Blocking One-P (PBS) was added to the microcentrifuge tube and suspended by pipetting.
- The microcentrifuge tube was incubated at room temperature for 30 min and centrifuged at 1,000 x g for 5 min.
- The supernatant was discarded, and 300 μl of Anti-PD1 antibody (Mouse) / 10% Blocking One-P (PBS) was added to the microcentrifuge tube and suspended by pipetting.
- The microcentrifuge tube was incubated at room temperature for 1 h and centrifuged at 1,000 x g for 5 min.
- The supernatant was discarded, and 300 µl of 10% Blocking One-P (PBS) was added to the microcentrifuge tube and suspended by pipetting.
- The microcentrifuge tube was centrifuged at 1,000 x g for 5 min.
- The supernatant was discarded, and 500 μl of Alexa FluorTM 488 Anti-Mouse antibody /10% Blocking One-P (PBS) was added to the microcentrifuge tube and suspended by pipetting.
- The microcentrifuge tube was incubated at room temperature for 1 h and centrifuged at 1,000 x g for 5 min.
- The supernatant was discarded, and 300 µl of 10% Blocking One-P (PBS) was added to the microcentrifuge tube and suspended by pipetting.
- The microcentrifuge tube was centrifuged at 1,000 x g for 5 min.
- The supernatant was discarded, and 300 µl of 10% Blocking One-P (PBS) was added to the microcentrifuge tube.
- The stained cells were passed through a cell strainer for a flow cytometer.
- The stained cells were analyzed using a flow cytometer (LSR-Fortessa X-20, Becton Dickinson).
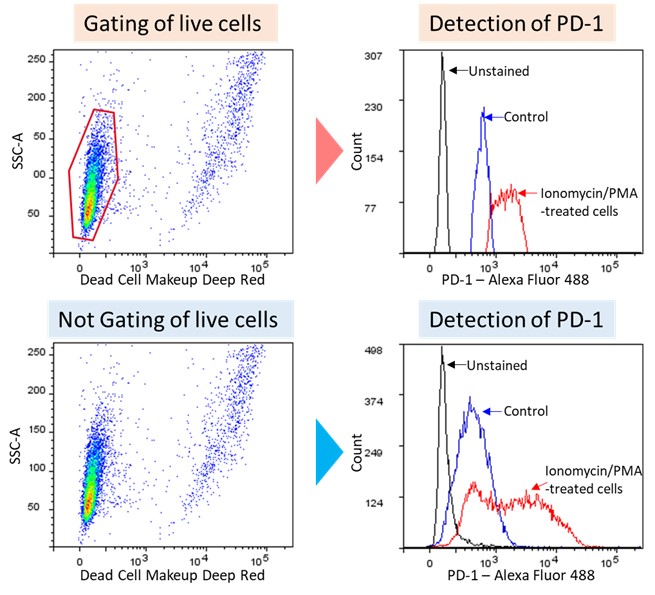
Figure3. PD-1 detection after exhaustion induction of MOLT-4 cells
C555_C556: Dead Cell Makeup Blue/Deep Red - Higher Retention than PI
Revised Aug., 30, 2023


 Hidden sections will not be printed.
Hidden sections will not be printed.