General Infomation
Cell surface antigens that are specifically expressed on cancer and immune cells have been actively studied for early detection and treatment of cancer. However, many cell surface proteins have low expression levels, and most of them are difficult to detect and analyze by conventional techniques.
A fluorescence detection method using a fluorescence-labeled antibody is widely known as a specific detection method for cell surface proteins (fluorescence immunostaining method). However, it is difficult to apply this method for low expressed surface proteins due to low sensitivity.
A highly sensitive CLAMP method (quinone methide-based catalyzed signal amplification) can be applicable to live/fixed cells or tissue sections. In this method, using β-galactosidase-labeled secondary antibody and newly developed fluorescent dye CLAMP F405, the cells expressing a specific cell surface protein are selectively and highly sensitively stained. CLAMP F405 allows highly sensitive detection of antigens by fluorescence imaging or flow cytometry.
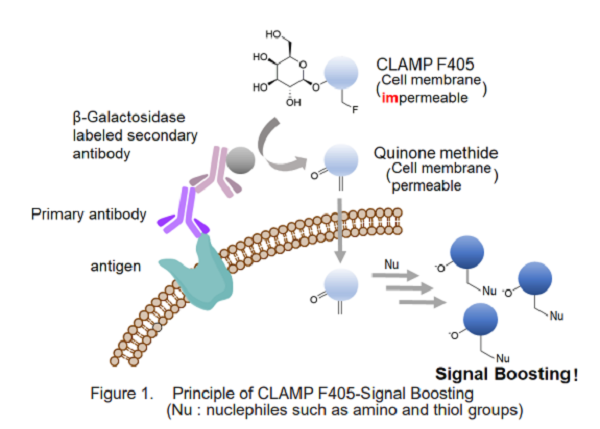
Kit Contents
CLAMP F405-Signal Boosting 10 µl x 1
Storage Condition
Store at -20°C
Required Equipment and Materials
-Isotype Control matched to a primary antibody
-β-Galactosidase-labeled secondary antibody
-CO2 incubator (37℃)
-Micropipette -1.5 ml microtube
-Hanks’ Balanced Salt Solution (HBSS)
-Phosphate buffered saline (PBS)
-Paraformaldehyde (PFA) solution -Blocking Buffer: Blocking Solution for fixed cell/tissue (ex:1% BSA/PBS, 10% Blocking One (03953-95: nacalai tesque)/PBS)
-Dimethyl sulfoxyde (DMSO: used for preparation of Staining Solution (Floating))
-Tube rotator (for staining floating cells, ex: RVM-101: scinics corporation)
Precaution
Centrifuge the tube briefly before opening to remove all content from walls of the tube and inside the cap.
Preparation of Solutions
Preparation of Staining Solution
The dilution rate of the solution varies depending on the type of sample. Prepare the solution according to the following procedure.
-Floating Cells-
Take CLAMP F405-Signal Boosting in a new 1.5 ml microtube and dilute 10-fold with DMSO. Dilute this solution 1,000-fold with HBSS (live cells) or PBS (fixed cells) to make Staining Solution (Floating).
Note: CLAMP F405-Signal Boosting diluted 10-fold with DMSO is stable at -20°C for 2 weeks after preparation.
Note: Staining Solution (Floating) solution cannot be stored and must be freshly prepared each day and used within the day.
-Adherent Cells or Tissue Sections-
Dilute CLAMP F405-Signal Boosting 1,000-fold with HBSS (live cells) or PBS (fixed cells or tissue sections) to make Staining Solution.
Note: Staining Solution cannot be stored and must be freshly prepared each day and used within the day.
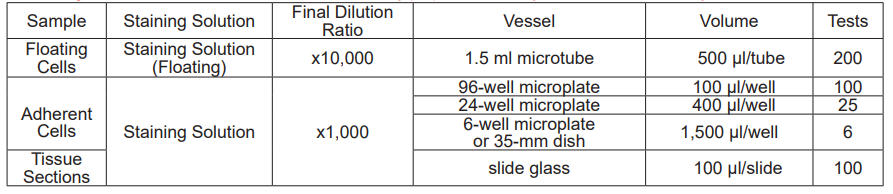
Selecting Protocol
Select the protocol according to the sample. Because of the high sensitivity of this technique, the background fluorescence will be high at the antibody concentrations used in general secondary antibody methods. Therefore, please consider (see [Considering proper antibody concentrations]) to set the appropriate antibody concentration for your sample in the protocol you choose.
Considering proper antibody concentrations
Perform all 8 conditions in the table below and select the one with sufficiently low fluorescence intensity in Isotype Control (conditions 1' - 4') and a high ratio to the fluorescence intensity in the corresponding conditions 1 - 4. (In the case of Figure 2, select conditions 3 and 3'.)
Note:The concentration of primary antibody should be lower than that recommended by the manufacturer.
The β-Galactosidase-conjugated secondary antibody should be diluted 500 - 10,000 times.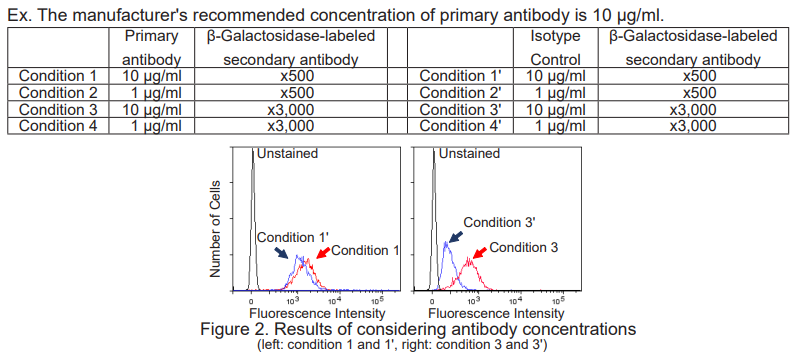
Protocol 1
For live and adherent cells

1. Inoculate cells into a microplate and incubate in a CO2 incubator at 37℃ overnight. 2. Discard the supernatant and wash the cells with 100 μl of medium twice.
3. Add 100 μl of primary antibody*/medium and incubate at 37°C for 60 minutes.
4. Discard the supernatant and wash the cells with 100 μl of medium twice.
5. Add 100 μl of β-Galactosidase-labeled secondary antibody*/medium and incubate at 37°C for 60 minutes.
6. Discard the supernatant and wash the cells with 100 μl of HBSS twice.
7. Add 100 μl of Staining Solution and incubate at 37°C for 30 minutes.
8. Discard the supernatant and wash the cells with 100 μl of HBSS three times.
9. Observe the cells under a fluorescence microscope.
*Consider proper antibody concentrations according to [Considering proper antibody concentrations].
Protocol 2
For live and floating cells
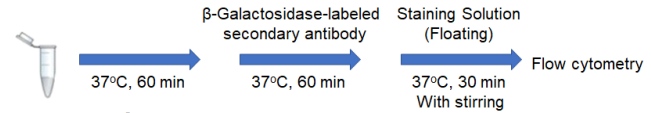
1. Prepare 2 x 105 cells/tube of a cell suspension in a 1.5 ml microtube.
2. Centrifuge at 300 × g for 5 minutes and discard the supernatant.
3. Add 500 μl of primary antibody*/medium and mix by pipetting.
4. Incubate at 37°C for 60 minutes.
5. Perform the following washing step:
I. Centrifuge at 300 x g for 5 minutes and discard the supernatant.
II. Add 500 μl of medium and mix by pipetting.
III. Centrifuge at 300 x g for 5 minutes and discard the supernatant.
IV. Perform steps II and III again.
6. Add 500 μl of β-Galactosidase-labeled secondary antibody*/medium and mix by pipetting.
7. Incubate at 37°C for 60 minutes.
8. Perform the same washing step as in step 5 with HBSS.
9. Add 500 μl of Staining Solution (Floating) and mix by pipetting.
10. Incubate at 37°C for 30 minutes with stirring using a tube rotator.
Note: If the cells are not suspended, the result might not be sensitive enough.
11. Centrifuge at 300 x g for 5 minutes and discard the supernatant.
12. Add 500 μl of HBSS and mix by pipetting.
13. Measure by a flow cytometer.
*Consider proper antibody concentrations according to [Considering proper antibody concentrations].
Protocol 3
For fixed and adherent cells
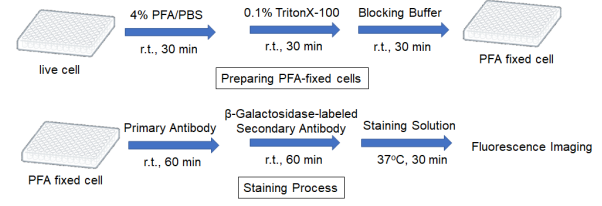
Preparing PFA fixed cells
1. Inoculate cells into a microplate and incubate in a CO2 incubator at 37℃ overnight.
2. Discard the supernatant and wash the cells with 100 μl of PBS twice.
3. Add 100 μl of 4% PFA/PBS and incubate at room temperature for 30 minutes.
4. Add 100 μl of 0.1% Triton-X100/PBS and incubate at room temperature for 30 minutes.
Note:If the target is a cell surface antigen, skip step 4.
5. Add 100 μl of Blocking Buffer and incubate at room temperature for 30 minutes.
Staining Protocol
1. Add 100 μl of primary antibody*/Blocking Buffer and incubate at room temperature for 60 minutes.
2. Discard the supernatant and wash the cells with 100 μl of Blocking Buffer twice.
3. Add 100 μl β-Galactosidase-labeled secondary antibody*/Blocking Buffer and incubate at room temperature for 60 minutes.
4. Discard the supernatant and wash the cells with 100 μl of Blocking Buffer twice.
5. Add 100 μl of Staining Solution and incubate at 37°C for 30 minutes.
6. Discard the supernatant and wash the cells with 100 μl of PBS three times.
7. Observe the cells under a fluorescence microscope.
*Consider proper antibody concentrations according to [Considering proper antibody concentrations].
Protocol 4
For fixed and floating cells
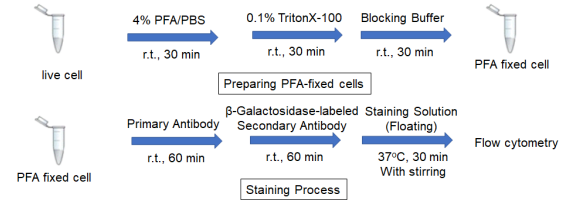
Preparing fixed cells
1. Prepare 2 x 105 cells/tube of a cell suspension in a 1.5 ml microtube.
2. Centrifuge at 300 × g for 5 minutes and discard the supernatant.
3. Add 500 μl of 4% PFA/PBS and mix by pipetting.
4. Incubate at room temperature for 30 minutes.
5. Centrifuge at 300 × g for 5 minutes and discard the supernatant.
6. Add 500 μl of 0.1% Triton-X100/PBS and mix by pipetting.
7. Incubate at room temperature for 30 minutes.
Note:If the target is a cell surface antigen, skip step 5-7.
8. Centrifuge at 300 × g for 5 minutes and discard the supernatant.
9. Add 500 μl of Blocking Buffer and mix by pipetting.
10. Incubate at room temperature for 30 minutes.
11. Centrifuge at 300 × g for 5 minutes and discard the supernatant.
12. Use the fixed cells for staining process.
Staining protocol
1. Add 500 μl of primary antibody*/Blocking Buffer and mix by pipetting.
2. Incubate at room temperature for 60 minutes.
3. Perform the following washing step:
I. Centrifuge at 300 x g for 5 minutes and discard the supernatant.
II. Add 500 μl of Blocking Buffer and mix by pipetting.
III. Centrifuge at 300 x g for 5 minutes and discard the supernatant.
IV. Perform steps II and III again.
4. Add 500 μl of β-Galactosidase-labeled secondary antibody*/Blocking Buffer and mix by pipetting.
5. Incubate at room temperature for 60 minutes.
6. Perform the same washing step as in step 3.
7. Add 500 μl of Staining Solution (Floating ) and incubate at 37°C for 30 minutes with stirring using a tube rotator.
Note:If the cells are not suspended, the result might not be sensitive enough.
8. Centrifuge at 300 x g for 5 minutes and discard the supernatant.
9. Add 500 μl of Blocking Buffer and mix by pipetting.
10. Measure by a flow cytometer.
*Consider proper antibody concentrations according to [Considering proper antibody concentrations].
Protocol 5
For frozen tissue
PFA fixation
1. Bring a frozen tissue section on a slide glass to room temperature.
2. Draw a border around the tissue section using a water-repellent pen.
3. Add 100 μl of 4% PFA/PBS and incubate at room temperature for 30 minutes.
Note: Use a humidity chamber not dry the sections during the following processes.
4. Discard the supernatant, add 100 μl of 0.1% Triton-X100/PBS and incubate at room temperature for 30 minutes.
5. Discard the supernatant, add 100 μl of Blocking Buffer and incubate at room temperature for 30 minutes.
Staining process
1. Discard the supernatant, add 100 μl of primary antibody*/ Blocking Buffer and incubate at room temperature for 60 minutes.
2. Discard the supernatant and wash with Blocking Buffer twice.
3. Add 100 μl of β-Galactosidase labeled antibody*/ Blocking Buffer and incubate at room temperature for 60 minutes.
4. Discard the supernatant and wash with Blocking Buffer twice.
5. Add 100 μl of Staining Solution and incubate at 37℃ for 30 minutes.
6. Discard the supernatant and wash with PBS three times.
7. Observe the cells under a fluorescence microscope.
*Consider proper antibody concentrations according to [Considering proper antibody concentrations].
Protocol 6
For formalin-fixed and paraffin-embedded (FFPE) tissue
Antigen Activation
1. Prepare FFPE tissue section on a slide glass.
2. Incubate at 60℃ for 60 minutes.
3. Incubate at room temperature for 15 minutes.
Note: Use a humidity chamber not dry the sections during the following processes.
4. Soak the slide with lemosol for 5 minutes. Repeat this step.
5. Soak the slide with ethanol for 5 minutes.
6. Soak the slide with 90% ethanol aqueous solution for 5 minutes.
7. Soak the silde with 80% ethanol aqueous solution for 5 minutes.
8. Wash the slide by soaking in ultrapure water, followed by PBS.
9. Perform the antigen activation.
10. Wash the slide by soaking in ultrapure water, followed by PBS.
Staining protocol
1. Draw a border around the tissue section using a water-repellent pen.
2. Add 100 μl of Blocking Buffer and incubate at room temperature for 30 minutes.
3. Discard the supernatant, add 100 μl of primary antibody*/Blocking Buffer and incubate at room temperature for 60 minutes.
4. Discard the supernatant, add 100 μl of PBS and incubate at room temperateure for 3 minutes. Repeat this step twice.
5. Add 100 μl of β-Galactosidase labeled secondary antibody*/ Blocking Buffer and incubate at room temperature for 60 minutes.
6. Discard the supernatant, add 100 μl of PBS and incubate at room temperature for 3 minutes. Repeat this step twice.
7. Add 100 μl of Staining Solution and incubate at 37℃ for 30 minutes.
8. Discard the supernatant, add 100 μl of PBS and incubate at room temperature for 3 minutes. Repeat this step twice.
9. Observe the cells under a fluorescence microscope.
*Consider proper antibody concentrations according to [Considering proper antibody concentrations].
Reference
K. Noguchi, T. Shimomura, Y. Ohuchi, M. Ishiyama, M. Shiga, T. Mori, Y. Katayama, and Y. Ueno, Bioconjugate Chemistry 2020 31(7), 1740-1744.
DOI: 10.1021/acs.bioconjchem.0c00180
Frequently Asked Questions / Reference
C554: CLAMP F405-Signal Boosting
Revised May., 18, 2023


 Hidden sections will not be printed.
Hidden sections will not be printed.

