General Information
-Bacstain- Bacterial Viability Detection Kits are series of products for fluorescent double-staining of bacteria. By combining different types of fluorescent stain, staining images can be acquired for each index (membrane impairment, respiratory activity, and esterase activity). Bacterial viability is generally assessed by colony formation in nutrient-agar medium. However, this requires a long culturing time, and it is hard to recognize the growth of viable but nonculturable (VNC) bacteria.
However, fluorescent staining does not require bacterial culture, and enables viability assessment by rapid and simple protocols.
-Bacstain- Bacterial Viability Detection Kit – DAPI/PI uses bacterial membrane damage as an index. DAPI is a minor groove binder specific to the AT sequence of DNA and permeates into bacteria to stain nucleic acids regardless of membrane damage. PI is a parallel intercalator into the DNA double helix that stains nucleic acid; it passes only through damaged bacterial membranes. Thus, this kit can be used to measure the ratio of membrane-damaged bacteria to total bacteria on analysis of the fluorescent images from each stain.
| Code | Product name | Combination |
| BS08 | -Bacstain- Bacterial Viability Detction Kit -DAPI/PI | Nucleic acids/Membrane impairment |
| BS09 | -Bacstain- Bacterial Viability Detction Kit -CTC/DAPI | Respiratory activity/Nucleic acids |
| BS10 | -Bacstain- Bacterial Viability Detction Kit -CFDA/PI | Esterase activity/Membrane impairment |
Kit Contents
| DAPI Solution | 25 μl × 4 |
| PI Solution | 25 μl × 4 |
Conditions
Store at ≤-20°C
Required Equipment and Materials
‐Incubator
‐Micropipettes (20 μl and 1,000 μl)
‐PBS(-) or saline
‐Fluorescence microscope
Precaution
Centrifuge the tube briefly before opening the cap because the contents may be on the tube wall or cap.
General Protocol
Each reagent included in this kit is sufficient for 100 samples.
- Equilibrate the DAPI and PI Solutions at room temperature by leaving the tubes for 30 min in the dark.
- Since PI is a potential mutagen, handling and disposal of this reagent should be considered with care.
- Adjust the bacterial suspension to 108–109 bacteria / ml with PBS(-) or saline.
- Transfer 1 ml of the bacterial suspension to a microtube.
- Centrifuge the suspension (5,000 x g, 5 min).
- Remove the supernatant, add 1 ml of PBS(-) or saline, and resuspend the bacteria.
- Repeat steps 4 and 5 twice.
- Add 1μl of DAPI Solution to the 1-ml bacterial suspension and vortex to mix well (final concentration of DAPI: 2.8 μmol/l).
- Incubate the bacterial suspension at room temperature for 5 min.
- Add 1 μl of PI Solution to the 1-ml bacterial suspension and vortex to mix well (final concentration of PI: 1.4 μmol/l).
- Incubate the bacterial suspension at room temperature for 5 min.
- Observe the bacteria under a fluorescence microscope.
| Component name | Maximum Ex/Em (nm) | Recommended filter | Excitation | Emission |
| DAPI Solution | 360/460 | DAPI | 320–400 nm | 410–510 nm |
| PI Solution | 530/620 | TRITC | 520–570 nm | 535–675 nm |
Experimental example-1
Double-staining of Staphylococcus aureus (a Gram-positive bacterium)
- Two test-tubes containing Staphylococcus aureus in 5 ml of liquid medium (TSBYE) were prepared and cultured at 37°C for 14–16 h. One tube was used as a live bacterial sample, and the other was autoclaved at 121 °C for 30 min to prepare a sterilized sample.
- The samples prepared in step 1 were diluted with PBS(-) to adjust the bacterial suspension to 108–109 bacteria/ml, then the live and sterilized samples were mixed at a ratio of 7:3.
- One milliliter of bacterial suspension was transferred to a microtube.
- The bacterial suspension was centrifuged at 5,000 x g for 5 min.
- The supernatant was removed and 1 ml of PBS(-) was added to the tube.
- Steps 4 and 5 were repeated twice.
- One microliter of DAPI Solution was added to the bacterial suspension and mixed well.
- The bacterial suspension was incubated at room temperature for 5 min.
- One microliter of PI Solution was added to the bacterial suspension and mixed well.
- The bacterial suspension was incubated at room temperature for 5 min.
- The bacteria were observed under an epi-illumination fluorescence microscope.
All bacteria are stained by DAPI(blue), bacteria with injured cell membrane are stained by PI(red).
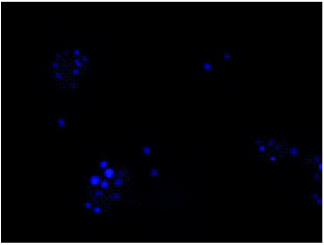
DAPI
Excitation filter 320–400 nm
Emission filter 410–510 nm
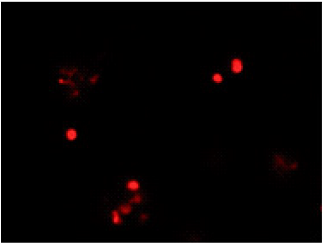
PI
Excitation filter 520–570 nm
Emission filter 535–675 nm
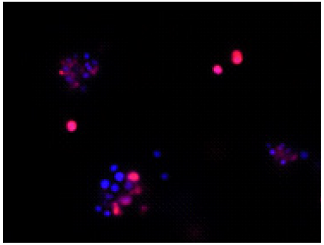
Merge
Experimental example-2
Double-staining of Escherichia coli (a Gram-negative bacterium)
- Two test-tubes containing Escherichia coli in 5 ml of liquid medium (TSBYE) were prepared and cultured at 37°C for 14–16 h. One tube was used as a live bacterial sample, and the other was autoclaved at 121°C for 30 min to prepare a sterilized sample.
- The samples prepared in step 1 were diluted with PBS(-) to adjust the bacterial suspension to 108–109 bacteria/ml, then the live and sterilized samples were mixed at a ratio of 1:1.
- One milliliter of bacterial suspension was transferred to a microtube.
- The bacterial suspension was centrifuged at 5,000 x g for 5 min.
- The supernatant was removed and 1 ml of PBS(-) was added to the tube.
- Steps 4 and 5 were repeated twice.
- One microliter of DAPI Solution was added to the bacterial suspension and mixed well.
- The bacterial suspension was incubated at room temperature for 5 min.
- One microliter of PI Solution was added to the bacterial suspension and mixed well.
- The bacterial suspension was incubated at room temperature for 5 min.
- The bacteria were observed under an epi-illumination fluorescence microscope.
All bacteria are stained by DAPI(blue), bacteria with injured cell membrane are stained by PI(red).
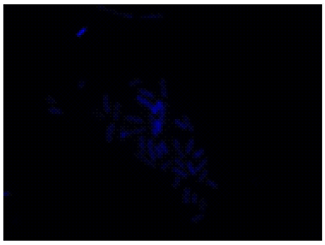
DAPI
Excitaition filter 320–400 nm
Emission filter 410–510 nm
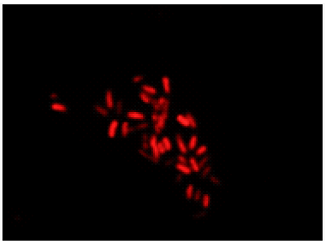
PI
Excitaition filter 520–570 nm
Emission filter 535–675 nm
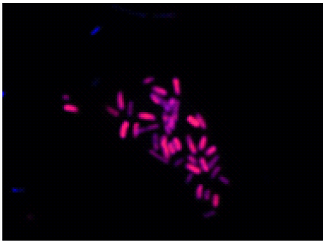
Merge
Frequently Asked Questions / Reference
BS08: -Bacstain- Bacterial Viability Detection Kit - DAPI/PI
Revised May., 22, 2023


 Hidden sections will not be printed.
Hidden sections will not be printed.