General Information
Adenosine triphosphate (ATP) is an energy source for living cells and is synthesized from its precursor adenosine diphosphate (ADP). The ATP concentration in cells is maintained by both glycolysis and mitochondrial oxidative phosphorylation. An Increase of the ADP/ATP ratio is recognized as a disruption of energy metabolism following apoptosis. The ADP/ATP ratio also changes in proliferating cells and during autophagy, which is a defense process against cellular stress. Recently, changes in the ADP/ATP ratio have been the focus of much attention in energy metabolism.
The ADP/ATP Ratio Assay Kit-Luminescence employs bioluminescent detection of the intracellular ADP and ATP. This kit enables easy detection of both ATP and ADP levels, with high sensitivity and determination of the ADP/ATP ratio.
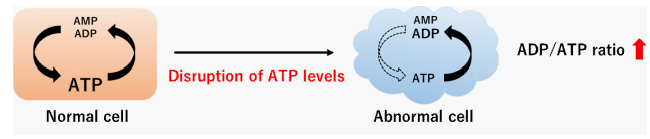
Figure 1. ADP/ATP ratio changes
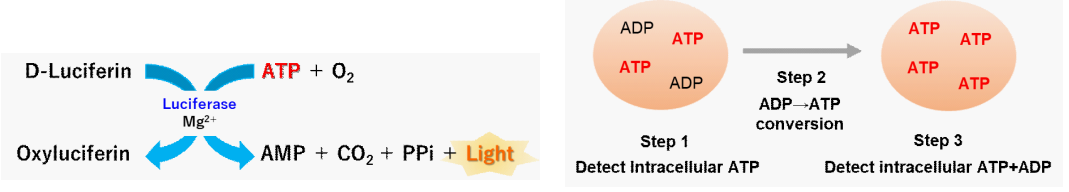
Figure 2. Principle of the ADP/ATP Ratio Assay Kit-Luminescence
Kit Contents
| ATP Enzyme Solution (Blue cap) | 20 μl x 1 |
| ATP Substrate | x 1 |
| Assay Buffer | 11 ml x 1 |
| ADP Enzyme Solution (Green cap) | 25 μl x 1 |
| ADP Substrate (Red cap) | x 1 |
| ADP Dilution Buffer (Clear cap) | 250 μl x 1 |
Storage Conditions
Store at 0-5 ℃
Required Equipment and Materials
- Microplate reader (luminescence)
- 96-well white microplate
- 20-200 µl multichannel pipette
- 100-1000 µl, 20-200 µl, 2-20 µl micropipettes
- 1.5 ml microtube
- Conical tube
Precautions
- Equilibrate reagents to room temperature prior to use.
- Briefly centrifuge the tubes containing the ATP Enzyme Solution and the ADP Enzyme Solution before opening to remove all content from the walls of the tube and the inside of the cap.
- Analysis of samples in triplicate is recommended for accuracy.
- In order to confirm linearity between the cell number and luminescence signal intensity, create a calibration curve and be constant ADP/ATP ratio before the assay.
- Following cell numbers are recommended in case using a 96-well microplate. Suspension cells (Jurkat cells):10,000–15,000 cells/well, Adherent cells (HeLa cells):4,000–5,000 cells/well
- The ATP Substrate is stored in a glass bottle with an aluminum cap. Handle with caution, wear gloves.
Preparation of Solutions
Preparation of ATP working solution
- Add 1 ml the Assay Buffer to the ATP Substrate vial.
- The ATP Substrate vials are capped under vacuum pressure. Please open it carefully. Do not open quickly to avoid spilling contents.
- Close the cap of the ATP Substrate vial and dissolve the contents completely and transfer it to the Assay Buffer bottle.
- Add 20 µl the ATP Enzyme Solution to the solution prepared in step 2.
- Centrifuge the ATP Enzyme Solution tube briefly before opening to remove all contents from the walls of the tube and inside the cap.
- Mix by gently inverting the contents to obtain a working solution.
- The ATP working solution is stable for 30 days when stored at -20 ℃.
Preparation of ADP Substrate working solution
Add 280 µl of double-distilled H2O (ddH2O) to the ADP Substrate tube and dissolve by pipetting.
- It is not easy to see the ADP Substrate tube. After adding double-distilled H2O (ddH2O), repeat pipetting several times and mix well.
- The ADP Substrate working solution is stable for 30 days when stored at 0-5 ℃.
Preparation of ADP Enzyme working solution
Add 25 µl the ADP Enzyme Solution and 225 µl the ADP Dilution Buffer to a new 1.5 ml microtube.
- Centrifuge the ADP Enzyme Solution tube briefly before opening to remove all contents from the walls of the tube and inside the cap.
- The ADP Enzyme working solution is stable for 30 days when stored at 0–5 ℃.
General Protocol
1. Sample preparation
<Suspension cells>
- Suspend cells in a medium containing a drug and seed them in a dish.
- ※ Prepare cells in the medium without the drug as a control.
- Incubate the cells in an incubator (37 ℃, 5% CO2).
- Add 10 µl cell suspension to separate wells in a 96-well white microplate.
- ※ To obtain accurate data, triplicate measurements are recommended.
<Adherent cells>
- Seed cells in a 96-well white plate.
- To obtain accurate data, triplicate measurements are recommended.
- Culture the cells overnight in an incubator (37 ℃, 5% CO2).
- Discard the supernatant and add a medium containing a drug.
- Incubate the cells in an incubator (37 ℃, 5% CO2).
- Remove the medium from the wells, taking care not to peel off the cells.
2. Assay
- Add 90 µl the ATP working solution to each well.
- Add the ATP working solution by using the process of reverse pipetting to avoid air bubbles that could influence the luminescent signal.
- After adding the ATP working solution, mix the contents for 2 minutes on an orbital shaker or similar.
- Protect the plate from light by covering it with aluminum foil because the luminescent signal is light sensitive.
- Incubate the microplate at 25 ℃ for 10 minutes in a microplate reader.
- Incubate the microplate in an incubator which has been temperature-settled at 25 ℃ or a room temperature around 25℃.
- The above incubation is necessary to efficiently extract ATP and ADP from cells and for a stable luminescent signal.
- Measure luminescence (Data 1) with a microplate reader.
- Refer to Table 1 for the preparation of the ADP working solution. Measure luminescence (Data 2) with a microplate reader immediately before addition.
- The ADP working solution cannot be stored and must be used within the day.
- This measurement provides the background prior to measuring ADP.
Table 1. Examples of ADP working solution preparation 2 samples
(6 wells)8 samples
(24 wells)16 samples
(48 wells)24 samples
(72 wells)32 samples
(96 wells)ADP Substrate
working solution15 μl 60 μl 120 μl 180 μl 240 μl ADP Enzyme
working solution15 μl 60 μl 120 μl 180 μl 240 μl - After measuring Data 2, immediately add 5 µl the ADP working solution to each well.
- After adding the ADP working solution, mix the contents for 2 minutes on an orbital shaker or similar.
- Incubate the microplate at 25 ℃ for 8 minutes in a microplate reader.
- Incubate the microplate in an incubator which has been temperature-settled at 25 ℃ or a room temperature around 25 ℃.
- The above incubation is necessary to efficiently convert intracellular ADP to ATP and for a stable luminescent signal.
- Measure luminescence (Data 3) with a microplate reader.
- Determine the ADP/ATP ratio using the equation.
ADP/ATP ratio = (Data 3 - Data 2)/Data 1
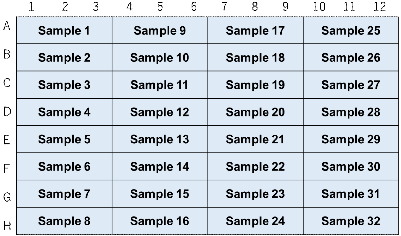
Figure 3. An example of plate arrangement (n=3)
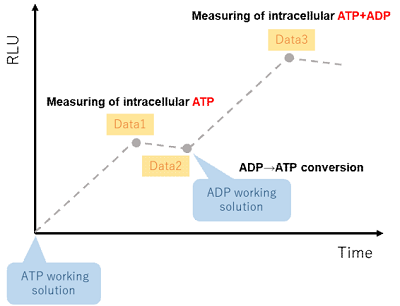
Figure 4. Outline of the measurement procedure
Experimental Example
Changes in intracellular ADP/ATP ratio by apoptosis induction using staurosporine
1. Sample preparation
- Jurkat cells (1.5×106 cells/ml) were suspended in a medium (RPMI containing 10% FBS) containing staurosporine (5 µmol/l), and the cells were seeded in a 5 cm dish (7.5×106 cells/dish).
- The cells were cultured for 5 hours in an incubator (37 ℃, 5% CO2).
- The cells were collected into a conical tube.
2. Measurement
- Cells suspensions (10 µl/well) were added to each well of a 96-well white microplate.
- The ATP working solution (90 µl/well) was added to each well.
- The microplate was orbitally shaken for 2 minutes in a microplate reader.
- The microplate was incubated at 25 ℃ for 10 minutes in a microplate reader.
- Luminescence (Data 1) was measured using a microplate reader.
- The ADP working solution was prepared, and luminescence (Data 2) was measured immediately using a microplate reader before adding the prepared ADP working solution.
- The ADP working solution (5 µl/well) was added to each well.
- The microplate was incubated at 25 ℃ for 8 minutes in a microplate reader.
- Luminescence (Data 3) was measured using a microplate reader.
- The ADP/ATP ratio was calculated as (Data 3 – Data 2)/Data 1.
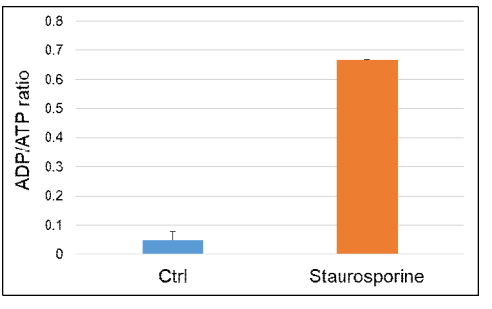
Figure 5. The intracellular ADP/ATP ratio was increased by the staurosporine treatment.
Frequently Asked Questions / Reference
A552: ADP/ATP Ratio Assay Kit-Luminescence
Revised Jun., 19, 2023


 Hidden sections will not be printed.
Hidden sections will not be printed.

