General Information
Adenosine triphosphate (ATP) is an energy source for living cells and is synthesized by both glycolysis and mitochondrial oxidative phosphorylation. Mitochondria generate 95% of cellular ATP, and mitochondrial dysfunction reduces ATP levels in cells. Therefore, the measurement of ATP levels has been established as a proxy for mitochondrial activity. Decreased ATP levels are associated with cancer, aging, neurodegenerative diseases, and mitochondrial diseases. Cancer cells rely on glycolysis, a process that is less efficient than oxidative phosphorylation to synthesize ATP. However, recent studies have revealed that ATP synthesis in cancer cells shifts from glycolysis to oxidative phosphorylation when glycolysis is suppressed1). The ATP Assay Kit-Luminescence enables the quantitation of intracellular ATP by luciferase luminescence assay. This kit includes an ATP standard to allow interexperimental comparisons. In addition, this kit provides a simple method that involves mixing the kit components and adding the reagent directly to cells cultured in a growth medium. Intracellular ATP concentration is measured without cell washing or medium removal. Furthermore, this kit can be applied for microplate assay.
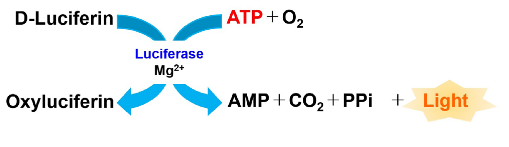
Figure 1 Principle of ATP Assay Kit-Luminescence
Kit Contents
| 50 tests | 100 tests | |
| Enzyme Solution | 10 μl x 1 | 20 μl x 2 |
| Substrate | x 1 | x 2 |
| Assay Buffer | 5.5 ml x 1 | 11 ml x 2 |
| ATP Standard | x 1 | x 1 |
Storage Conditions
Store at −20 ℃
Required Equipment and Materials
- Serum-free medium
- Microplate reader (luminescence)
- 96-well white microplate
- If ATP levels in adherent cells are measured, select a cell culture plate.
- If microscopic observation of cells or bottom reading measurement of a microplate is required, use a white plate with clear-bottom.
- Refer to the Q&A for tested white plates.
- 20–200 µl multichannel pipette
- 100–1000 µl and 20–200 µl micropipettes
- Conical tube
Precautions
- Equilibrate reagents to room temperature prior to use.
- Centrifuge the tube with Enzyme solution and ATP Standard briefly before opening to remove all content from walls of the tube and inside the cap.
- Analyzing samples and standards in triplicate is recommended for accuracy.
- Prepare samples at different dilutions to determine the most suitable concentration, ranging from 0–2.5 µmol/l ATP.
- Adjust the number of cells less than 10,000 cells/well when 96-well microplate is used. This appropriate number of cells can be applied for both adherent and suspension cells.
- Substrate is stored in a glass bottle with an aluminum cap. Handle with caution, wear gloves.
Preparation of Solutions
Preparation of Working solution
- Add 1 ml of Assay Buffer to the Substrate vial.
- Substrate vials are capped under vacuum pressure. Please open it carefully. Do not open quickly to avoid spilling contents.
- Close the cap of the Substrate and dissolve the contents completely and transfer it to the Assay Buffer bottle.
- Add Enzyme Solution to the solution prepared in step 2.
- 50 tests : Add 10 µl of Enzyme solution to the 5.5 ml of Assay Buffer to prepare a Working solution.
200 tests : Add 20 µl of Enzyme solution to the 11 ml of Assay Buffer to prepare a Working solution. - Centrifuge the Enzyme solution tube briefly before opening to remove all contents from walls of the tube and inside the cap.
- 50 tests : Add 10 µl of Enzyme solution to the 5.5 ml of Assay Buffer to prepare a Working solution.
- Mix by gently inverting the contents to obtain a Working solution.
- The Working solution is stable for 30 days when stored at −20 ℃
Preparation of ATP stock solution
Add 200 µl of double-distilled H2O (ddH2O) to the ATP Standard tube and dissolve by pipetting to prepare a 1 mmol/l ATP stock solution.
- Centrifuge the ATP Standard tube briefly before opening to remove all contents from the walls of the tube and inside the cap.
- The ATP stock solution is stable for 10 days when stored at −20 ℃
General Protocol
1. Sample preparation
Add 100 µl of a cell suspension to a 96-well white microplate.
- Analyzing samples and standards in triplicate is recommended for accuracy.
- Prepare samples at different dilutions to determine the most suitable concentration, ranging from 0–2.5 µmol/l ATP.
2. Preparation of ATP standard solution
- Mix 10 µl of 1 mmol/l ATP stock solution and 990 µl of serum-free medium in a conical tube to prepare a 10 µmol/l ATP standard solution.
- ※ Please use serum-free medium with the same composition as the culture medium. Endogenous ATPase in sera such as fetal bovine serum (FBS) may reduce the luminescent signal.
- Mix 250 µl of 10 µmol/l ATP standard solution and 750 µl of serum-free medium in a conical tube to prepare a 2.5 µmol/l ATP standard solution.
- Prepare the following ATP standard solutions by serial dilution with serum-free medium: 2.5, 1.25, 0.625, 0.313, 0.156, 0.078, 0.039 and 0 µmol/l (Figure 2).
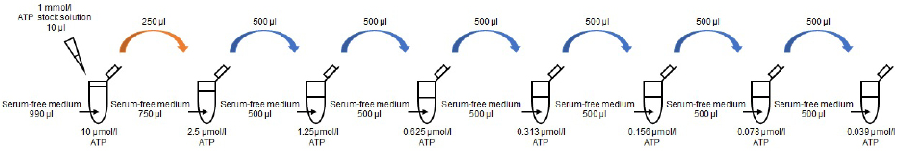
Figure 2 Preparation of ATP standard solutions
3. Measurement
- Add 100 µl of the varying concentrations of ATP standard solutions to each well (Figure 3).
- Add 100 µl of Working solution to each well.
- Mix the contents by using the process of reverse pipetting to avoid air bubbles that could influence the luminescent signal.
- After adding the Working solution, mix the contents for 2 minutes on an orbital shaker or similar.
- Please protect the plate from light by covering with aluminum foil because the luminescent signal is light sensitive.
- Incubate the microplate at 25 ℃ for 10 minutes in a microplate reader.
- Please incubate the microplate in an incubator which has been temperature-settled at 25 ℃ or at a room temperature around 25 ℃.
- The above incubation is necessary to efficiently extract ATP from cells and for a stable luminescent signal.
- Measure luminescence with a microplate reader.
- Calculate the ATP concentration in each sample from a calibration curve generated from the ATP standards.
-
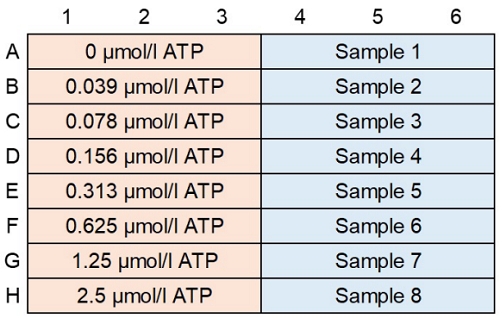
Figure 3 An example of plate arrangement (n=3)
-
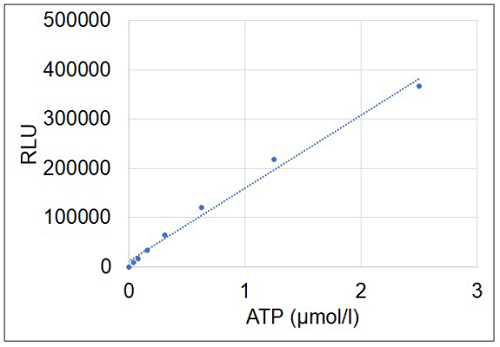
Figure 4 Typical calibration curve generated with ATP standards.
Experimental Example
Changes in intracellular ATP level due to electron transport chain inhibition using Rotenone
1. Sample preparation
- Jurkat cells were seeded (2×105 cells/well, in RPMI containing 10% FBS) in two wells of a 6-well plate.
- Rotenone solution (1 ml, 50 µmol/l in RPMI containing 10% FBS) was added in one well of the 6-well plate, and RPMI containing 10% FBS (1 ml) was added to another well.
- The cells were cultured for 4 hours in an incubator (37 ℃, 5% CO2).
2. Measurement
- Sample and ATP standard solutions (100 μl each) were added to individual wells of a 96-well microplate.
- The working solution (100 μl) was added to each well(Figure 5).
- The microplate was orbitally shaken for 2 minutes in a plate reader.
- The microplate was incubated at 25 ℃ for 10 minutes in a microplate reader.
- Luminescence was measured using a plate reader, and the concentration of ATP in the samples was calculated from the calibration curve.
-
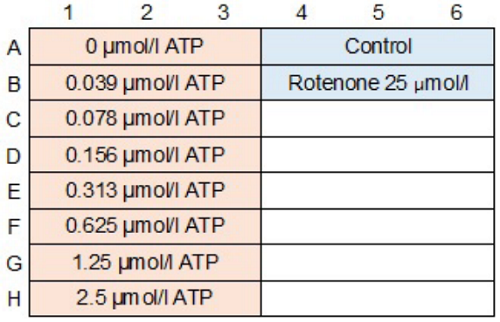
Figure 5 An example of plate arrangement (n=3)
-
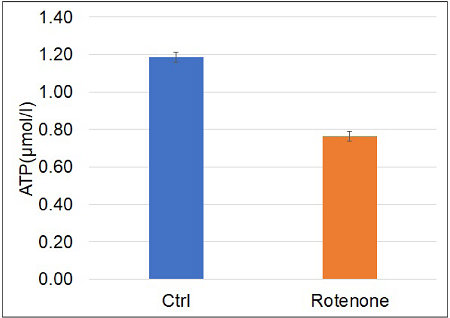
Figure 6 Intracellular ATP levels were decreased by the electron transport chain inhibitor rotenone
Reference
- R. Shiratori, et al., Sci. Rep., 2019, 9, 18699.
Frequently Asked Questions / Reference
A550: ATP Assay Kit-Luminescence
Revised Apr., 11, 2024


 Hidden sections will not be printed.
Hidden sections will not be printed.