Fura 2-AM

Reagent for Monitoring Intracellular Calcium Ion
-
Product codeF015 Fura 2-AM
-
CAS No.108964-32-5
-
Chemical name1-[6-Amino-2-(5-carboxy-2-oxazolyl)-5-benzofuranyloxy]-2-(2-amino-5-methylphenoxy)ethane-N,N,N',N'-tetraacetic acid, pentaacetoxymethyl ester
-
MWC44H47N3O24=1001.85
| Unit size | Price | FUJIFILM Wako Products code |
|---|---|---|
| 1 mg | ¥37,200 | 341-08621 |
Precautions:
This product is typically attached to the bottom of the tube in a film-like state.
In rare cases, due to vibrations during transportation, the product may detach from the bottom of the tube and adhere to the tube wall or the back of the cap.
Please open the package and allow the contents to fall out before use.
If you have any questions, please contact us here.
Description
Product Description
Fura 2 was developed to improve the fluorescent properties of Quin 2. The signal intensity in 1 mM of loaded Fura 2 corresponds to that of 30 mM of loaded Quin 2. This allows an experiment at a lower concentration of indicator using Fura 2 instead of Quin 2. Fura 2 is one of the most widely used calcium indicators for ratiometric measurement. Many types of instrumentation are now available for experiments using Fura 2, but Fura 2 is especially suitable for digital imaging microscopy. It is less susceptible to photobleaching than Indo 1. Changes in the cell shape can sometimes affect the fluorescent ratio at 340 nm and 380 nm. For example, fluorescent signal intensities at these wavelengths sometimes decrease simultaneously with smooth muscle contraction. For blood vessels, however, the increase of the signal intensity at 340 nm tends to be smaller on contraction, while the decrease of the signal intensity at 380 nm tends to be larger with its contraction. Fura 2-AM is an acetoxymethyl ester derivative of Fura 2 that can be easily loaded into cells by incubation.
Hydrolysis of AM ester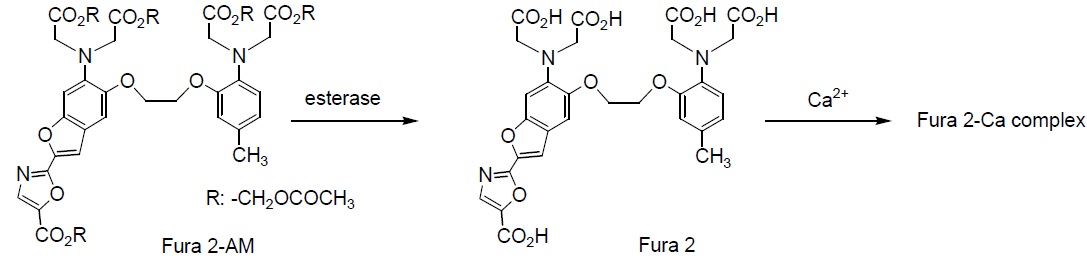
Technical info
General Protocol (for NG 108-15/ Neuron Cell Line)*
Reagents:
・1 mM Fura 2-AM/DMSO (1 mg Fura 2-AM in 1 ml DMSO)
・Hanks Ebalanced salt solution (HBSS)
・HEPES buffer saline (20 mM HEPES, 115 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 0.8 mM MgCl2, 13.8 mM glucose, pH 7.4)
Protocol:
1. Culture cells on a glass-bottom dish using DMEM containing 5% fetal calf serum.
2. Change the medium to 1 mM dibutyl cAMP/DMEM, and culture the cells for 3-4 days to induce dendrites.
3. Dilute 20 μl of 1 mM Fura 2-AM DMSO solution with 20 ml of HEPES buffer saline to prepare 1 μM Fura 2-AM working solution.
*Keep the Fura 2-AM working solution warm at 37oC. Make sure to disperse Fura 2-AM in the solution by a ultrasonication or an addition of Chremophor® EL or Pluronic® F127 (final conc.: 0.02%).
4. Remove the culture medium, and add 0.5 ml of the Fura 2-AM working solution to the cells.
5. Incubate for 20 minutes. Then remove the Fura 2-AM working solution.
6. Wash the cells once with HEPES buffer saline. Then incubate the cells for 1 hour in the HEPES buffer saline.
7. Use the cells for fluorescent calcium ion detection.
8. Monitor the excitation spectra at 380 nm (calcium free) and 340 nm (calcium complex) with fixed emission at 510 nm.
*Cell staining conditions differ by cell types, so it is necessary to optimize the conditions for each experiment.
References
1. G. Grynkiewicz, et al., A New Generation of Ca2+ Indicators with Greatly Improved Fluorescence Properties. J Biol Chem. 1985;260:3440-3450.
2. D. A. Williams, et al., Calcium Gradients in Single Smooth Muscle Cells Revealed by the Digital Imaging Microscope Using Fura-2. Nature. 1985;318:558-561.
3. R. Y. Tsien, et al., Measurement of Cytosolic Free Ca2+ in Individual Small Cells Using Fluorescence Microscopy with Dual Excitation Wavelengths. Cell Calcium. 1985;6:145-157.
4. D. A. Williams, et al., Intracellular Calibration of the Fluorescent Calcium Indicator Fura-2. Cell Calcium. 1990;11:75-83.
5. W. Almers, et al., The Ca Signal from Fura-2 Loaded Mast Cells Depends Strongly on the Method of Dye-loading. FEBS Lett. 1985;192:13-18.
6. G. H. Rao, et al., Measurement of Ionized Calcium in Blood Platelets with a New Generation Calcium Indicator E Biochem Biophys Res Commun. 1985;132:652-657.
7. H. Ozaki, et al., Simultaneous Recordings of Calcium Signals and Mechanical Activity Using Fluorescent Dye Fura 2 in Isolated Strips of Vascular Smooth Muscle. Jpn J Pharmacol. 1987;45:429-433.
8. M, Mitsui, et al., Leakage of the Fluorescent Ca2+ Indicator Fura-2 in Smooth Muscle. Jpn J Pharmacol. 1993;61:165-170.
Q & A
-
Q
Several types of intracellular calcium measurement reagents are available. How do I choose the right one?
-
A
The selection is often based on the measurement instruments and wavelengths used.
Products with "[-AM]" in their names are cell membrane-permeable.
The characteristics of each reagent are as follows:【Fura-2】
• Two excitation, one fluorescence
Excitation: λex = Ca: 340 nm, Ca-free: 380 nm; fluorescence: λem = 500 nm.
• Dissociation constant: 224 nmol/L
• The measurement is based on the fluorescence intensity ratio, which allows for correction of error factors.
This facilitates calculation of intracellular Ca²⁺ concentration.
• This is the most widely used method.
• Requires switching excitation filters, resulting in time loss between switches.
(Depends on instrument performance.)【Fluo 3】
• One excitation, one fluorescence
Excitation: λex = 508 nm; fluorescence: λem = 527 nm.
• Dissociation constant: 400 nmol/L
• The excitation wavelength is in the longer wavelength range, which reduces cellular damage.
(Not affected by NADH or NADPH).
• Can be used with an argon laser excitation device.
• Not suitable for slice specimens.
Does Fluo 3 bind to dead cells on the slice surface or measure extracellular calcium concentration?【Fluo 4】
• One excitation, one fluorescence
Excitation: λex = 495 nm; fluorescence: λem = 518 nm.
• Dissociation constant: 345 nmol/L
Compared to Fluo 3, the excitation peak wavelength is shifted approximately 10 nm toward shorter wavelengths, resulting in approximately twice the fluorescence intensity and higher sensitivity when excited by an argon laser.【Indo 1】
• One excitation, two fluorescence.
Excitation: λex = 330 nm; fluorescence: λem = Ca: 410 nm, Ca-free: 485 nm.
• Dissociation constant: 250 nmol/L
No need to switch excitation wavelengths, enabling measurement of samples with very fast Ca²⁺ concentration changes or moving samples, such as cardiac muscle cells.
(However, two detectors are required.)【Rhod 2】
1 excitation, 1 fluorescence
Excitation: λex = 553 nm; fluorescence: λem = 576 nm
• Dissociation constant: 1.0 μmol/L
Since the excitation wavelength is on the long wavelength side, it causes minimal cell damage.
(no influence from NADH or NADPH).
• Can be used with an argon laser excitation device.【Quin 2】
• One excitation, one fluorescence
Excitation: λex = 339 nm; fluorescence: λem = 492 nm.
• Dissociation constant: 110 nmol/L
• First probe developed.
-
Q
This is my first time performing this measurement. Could you please explain the principle, measurement method, and troubleshooting methods for Ca²⁺ probes in an easy-to-understand manner?
-
A
We have created a protocol for individuals performing intracellular Ca²⁺ measurements for the first time.
The protocol includes information on the principles, characteristics of Ca²⁺ probes, measurement methods, and troubleshooting methods. Please refer to it.
The protocol can be downloaded from the link below.For those who are measuring intracellular Ca2+ for the first time
Handling and storage condition
| Appearance: | Yellow to yellowish orange crystalline powder or solid |
|---|---|
| Purity (HPLC): | ≧ 98.0 % |
| Fluorescence spectrum: | To pass test |
| NMR spectrum: | Authentic |
| -20°C, Protect from light |









