Intracellular Oxygen Detection Kit
Intracellular Oxygen Detection Kit
- Measeure intracellular oxygen concentration by phosphorescence detection
- Detectable using a fluorescence microscope or a plate reader that supports temperature control and time-resolved fluorescence measurement.
- Mineral oil included in kit to prevent influx of oxygen from the air.
-
Product codeI306 Intracellular Oxygen Detection Kit
| Unit size | Price | Item Code |
|---|---|---|
| 100 tests | Find your distributors | I306-10 |
| 300 tests | Find your distributors | I306-12 |
| 100 tests | Intracellular Oxygen Probe Mineral Oil |
×1 10 ml×1 |
|---|---|---|
| 300 tests | Intracellular Oxygen Probe Mineral Oil |
×3 10 ml×3 |
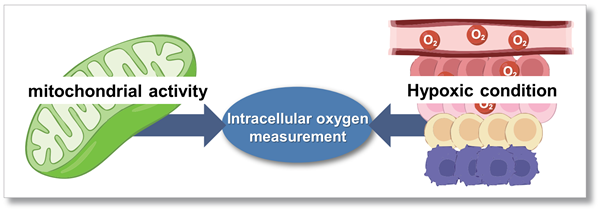
To detect mitochondrial activity and hypoxic condition
Aerobic organisms mainly produce ATP by mitochondrial oxidative phosphorylation; because oxygen (O2) is consumed in this process, it is one of the substances that is essential for energy production. Intracellular oxygen levels are also known as an important indicator because chronic hypoxia in cells leads to tumour progression and ischaemic diseases.
Manual
Technical info
This kit contains the Intracellular Oxygen Probe, which has the property of increasing phosphorescence intensity as the oxygen concentration in the cell decreases, and Mineral Oil to block the influx of oxygen from the air into the well. The phosphorescence intensity corresponding to the oxygen concentration in the cell can be detected with a fluorescence microplate reader or fluorescence microscope by simply adding the reagents. For measurement with a fluorescent microplate reader, the phosphorescence lifetime is calculated based on the formula described in the instruction manual.
*Measurement of intracellular oxygen concentration with a plate reader requires a plate reader capable of temperature control and time-resolved fluorescence measurement.
This product was developed by Professor Yoshihara et al. at the Graduate School of Science and Technology, Gunma University.
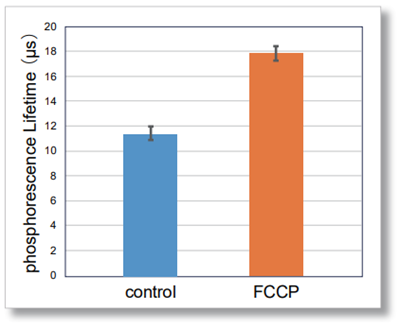
Changes in intracellular oxygen by FCCP treatment
Intracellular oxygen changes (phosphorescence lifetime) were measured in HepG2 cells treated with FCCP, which increases mitochondrial oxygen consumption, for 1 hour using a fluorescent microplate reader. The results showed that the phosphorescence lifetime increased in FCCP-treated cells compared to control cells, confirming a decrease in intracellular oxygen.
-
[Detection conditions]
Plate reader: TECAN InfinitePRO 200, bottom reading[Experimental conditions]
Cell type: HepG2 cells(7.2×104 cell/well)
FCCP concentration: 4 µmol/l
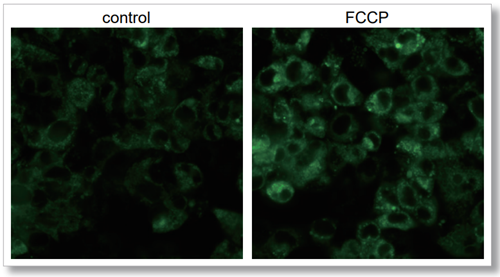
Imaging analysis with fluorescence microscope
HepG2 cells seeded in triple-well glass base dishes (IWAKI, code: 3970-103) were treated with FCCP, which increases mitochondrial oxygen consumption, for 2 hours, and the intracellular oxygen changes were analyzed by imaging with this product. The increase in Intracellular Oxygen Probe signal was observed in FCCP-treated cells compared to control cells, indicating that the decrease in intracellular oxygen was detected.
-
[Detection conditions]
Confocal fluorescence microscope(ZEISS, LSM800)
Ex/Em = 488/550-700 nm
Laser: 1.0%, 700V
Lens: ×40
Scan speed: 3
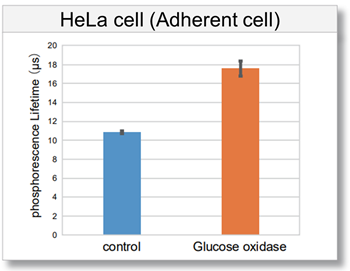
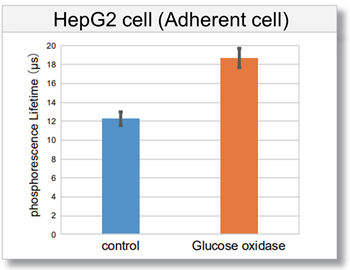
Detection of intracellular hypoxic condition by Glucose oxidase treatment
Intracellular oxygen changes (phosphorescence lifetime) of three types of cells (HeLa cells, HepG2 cells, and Jurkat cells) treated with Glucose oxidase, which consumes dissolved oxygen in the medium and induces a hypoxic condition, were measured using a fluorescent microplate reader. As a result, the phosphorescence lifetime of the Glucose oxidase-treated cells increased compared to the control cells, confirming the decrease in intracellular oxygen.
[Detection conditions]
Plate reader: TECAN InfinitePRO 200, bottom reading
[Experimental conditions]
Cell type: HeLa cell (7.8×104 cell/well), HepG2 cell (7.2×104 cell/well), Jurkat cell (1.0×105 cell/well)
Glucose oxidase concentration: 0.5 U/ml
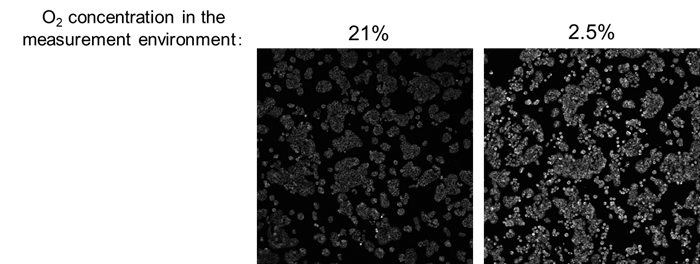
Detection of Oxygen Responsiveness in Hypoxic Environments
We performed imaging analysis of changes in intracellular oxygen in HT-29 cells under varying oxygen concentrations in the measurement environment.
As a result, an increase in Intracellular Oxygen Probe signal was observed under hypoxic condition (2.5%) compared to normoxic condition (21%), which corresponds to the oxygen concentration in the atmosphere.
[Detection conditions]
Confocal laser scanning microscope (Evident, FV3000)
Ex/Em = 488/560-660 nm 20 µs/pixel (number of pixels: 512 x 512, 2.486 µm/pixel)
Data provided by: Professor Yoshihara at the Graduate School of Science and Technology, Gunma University.
Q & A
-
Q
How many samples can be tested by this kit?
-
A
When testing one cell type with the same number of cells, 30 samples can be measured in 100 tests and 90 samples can be measured in 300 tests.
*If more than two cell types or multiple cell numbers are used in an experiment, separate Blanks and Controls must be prepared, and the number of samples that can be measured will vary. For details, please refer to the examples of plate layouts according to the manual.
-
Q
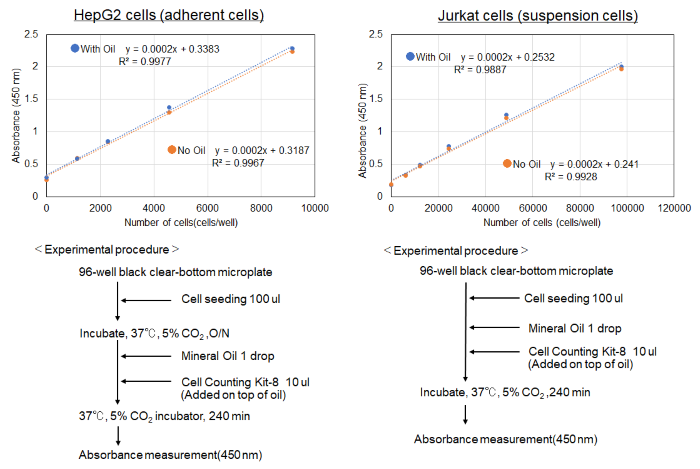
Does Mineral Oil have cytotoxicity to cells?
-
A
No toxicity was observed in cells treated with Mineral oil when measured by Cell Counting Kit-8 cytotoxicity assay.
(Reference)
-
Q
Can I store the working solution for a long period of time?
-
A
The working solution cannot be stored. Prepare it as needed.
-
Q
Will repeated freezing and thawing of Intracellular Oxygen Probe or Mineral Oil affect the assay?
-
A
We have confirmed that repeated freeze-thaw cycles of Intracellular Oxygen Probe and Mineral Oil have no effect on the assay.
-
Q
Why temperature-controllable plate readers are highly recommended for this kit?
-
A
If the microplate is incubated with an incubator (or heat block, thermostatic chamber, etc.) after adding reagents and Mineral Oil, the temperature difference in the plate reader will prevents accurate measurement of changes in intracellular oxygen concentration. This leads to a decrease in reproducibility. Therefore, please use a temperature-controllable plate reader.
<General Protocol>
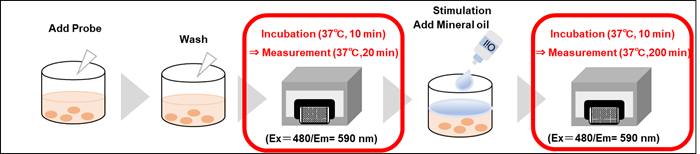
Step 7 and 12 for suspension cells; Step 7 and 12 for adherent cells in the manual.
-
Q
Can Top Reading measurement is applicable?
-
A
Top reading measurement is not recommended due to the influence of Mineral oil. Please measure from bottom reading.
-
Q
Are there any applications for suspension cells using a plate reader?
-
A
We prepared an example of Jurkat cells experiment.
<Protocol>
(1) Jurkat cells (1.0×106 cells/ml) were suspeded in working solution in a conical tube.
(2) The cell suspension (1) was transfferd to a culture vessel and culture in an incubator (37 °C, 5% CO2) for 1 h.
(3) The cell suspension (2) was collected in a conical tube and centrifuged (1500 rpm, 3 min) to remove the supernatant.
(4) RPMI medium (containing 10% FBS) was added to the conical tube, the cells were thoroughly suspended, centrifuged again (1500 rpm, 3 min), and the supernatant was removed.
(5) Cells without Intracellular Oxygen Probe (Blank) and cells with Probe (control and Glucose oxidase) were suspended in RPMI medium (containing 10% FBS) and seeded into 96-well black clear bottom plates at 100 µl (1.0×105 cells/well) in 96-well black clear-bottom plates.
(6) The microplate was placed in a plate reader pre-set at 37°C and incubated for 10 minutes.
(7) Kinetics were measured using the microplate reader (Ex: 480 nm, Em: 590 nm, 10-min interval for 20 min, Bottom reading, two conditions).
(8) The microplate was taken out of the microplate reader and added 10 µl of RPMI medium (containing 10% FBS) to Blank and Control wells.
(9) RPMI medium (containing 10% FBS) was added to the Blank and Control wells, and 10 µl of Glucose oxidase (5 U/ml) diluted in RPMI medium (containing 10% FBS) was added to each well in Sample 1.
(10) One drop of Mineral Oil was added to each well immediately after the addition of the Sample solution.
(11) The microplate was placed in a plate reader set at 37°C and incubated for 10 minutes.
(12) Kinetics were measured using the microplate reader (Ex: 480 nm, Em: 590 nm, 10-min interval for 200 min, Bottom reading, two conditions).
(13) Phosphorescence lifetime values were calculated from the intensity measurements.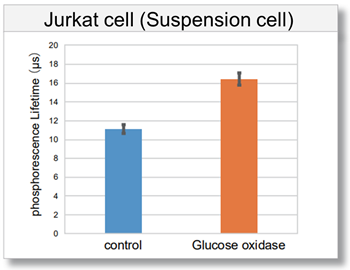
-
Q
Can I use the serum-free medium?
-
A
The measurement is possible. However, the results may differ from serum-containing medium. To compare the results, perform the assay under the same medium conditions each time.
Handling and storage condition
| Store at -20 °C, protect from light |