Previous Science Note
| Aging can alter mitochondrial activity in cells and change quantifiable features such as membrane potential and cristae structure. Such mitochondrial states can influence cellular fitness and senescence-associated programs. Recent studies show that aging expands Dnmt3a-mutant blood stem cell clones; their abnormally high mitochondrial activity enables them to outcompete normal stem cells, accumulate as pro-inflammatory cells, and increase the risk of age-related diseases. Another study reports that DNA damage induces phosphorylation of the mitochondrial membrane protein BNIP3, increases cristae and fatty acid oxidation, and drives histone acetylation with induction of p16 expression. These results indicate that changes in mitochondrial state are associated with aging-related phenotypes and the induction of senescence markers. | ||||||||||||||||||||
|
Elevated mitochondrial membrane potential is a therapeutic vulnerability in Dnmt3a-mutant clonal hematopoiesis (Nature Communications, 2025) Highlighted technique: To evaluate mitochondrial function in HSPCs/HSCs, the authors immunophenotyped freshly isolated bone marrow cells, measured mitochondrial membrane potential using TMRE-based detection, and further assessed it using additional mitochondrial membrane potential probes and mitochondrial Ca²⁺ indicators. |
||||||||||||||||||||
|
Mitochondrial fatty acid oxidation drives senescence (Science Advances, 2024) Highlighted technique: This study assessed senescence using multiple markers, including p16 expression and SA-βgal activity. While other markers changed, γH2AX did not, reinforcing the widely accepted notion that no single marker defines senescence. Thus, multiple indicators are needed to assess senescence. |
||||||||||||||||||||
Senescence and Mitochondrial Indicators (click to open/close)
|
||||||||||||||||||||
Application Note (click to open/close)
|
||||||||||||||||||||
|
|
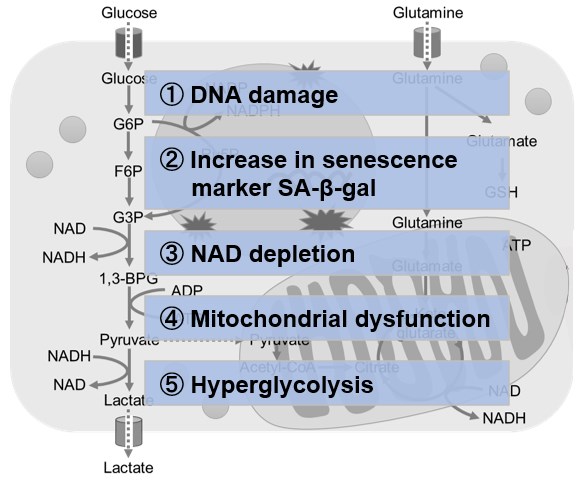
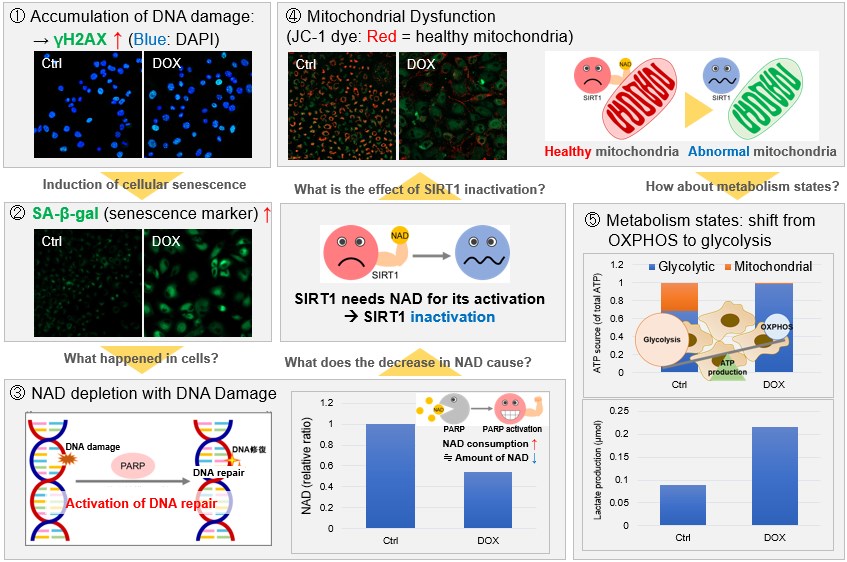
NAD(+) levels decline during the aging process, causing defects in nuclear and mitochondrial functions and resulting in many age-associated pathologies*. Here, we try to redemonstrate this phenomenon in the doxorubicin (DOX)-induced cellular senescence model with a comprehensive analysis of our products. *S. Imai, et al., Trends Cell Biol, 2014, 24, 464-471
|
 |
|