| A key task in aging research is to find senescent or dysfunction-shifted cells that hide among otherwise normal cells and to understand their properties. Many aging markers have already been reported, yet their expression can vary by cell type; alongside broad markers, newer work is uncovering markers tailored to specific lineages. This piece introduces two recent studies that propose characteristic markers for such aging-associated cells. In neurons, ferritin light chain 1 (FTL1) is presented as a candidate brain aging marker linked to iron redox balance, with suggestive connections to synaptic function and cognition. In the immune system, surface KLRG1 serves as a practical handle to detect a Treg subset that increases with age and to capture features such as metabolic strain and reduced suppressive capacity. | ||||||||||||||||||||||
|
Targeting iron-associated protein Ftl1 in the brain of old mice improves age-related cognitive impairment (Nature Aging, 2025) Highlighted technique: To visualize the redox state of iron in hippocampal tissue, the study used DNAzyme probes in which a fluorophore and a quencher are linked by nucleic acid, and when the DNA reacts at a specific substrate site it is cleaved and the probe lights up. Two probes were applied, one selective for Fe2+ and one for Fe3+, and the resulting images were combined to map the Fe3+/Fe2+ ratio and distinguish oxidized and reduced regions in the tissue. |
||||||||||||||||||||||
|
KLRG1 identifies regulatory T cells with mitochondrial alterations that accumulate with aging (Nature Aging, 2025) Highlighted technique: The study shows that KLRG1⁺ regulatory T cells, which accumulate with age, display higher levels of aging markers (p16, p21, γH2AX) and mitochondrial dysfunction, evidenced by reduced membrane potential with fluorescent probes and cristae abnormalities by electron microscopy. In a co-transfer assay into T-cell–deficient mice, these KLRG1⁺ Treg provided weaker protection than KLRG1⁻ Treg, establishing them as a functionally impaired population linked to age-related inflammation. |
||||||||||||||||||||||
Senescence Indicators (click to open/close)
|
||||||||||||||||||||||
Application Note (click to open/close)
|
||||||||||||||||||||||
|
|
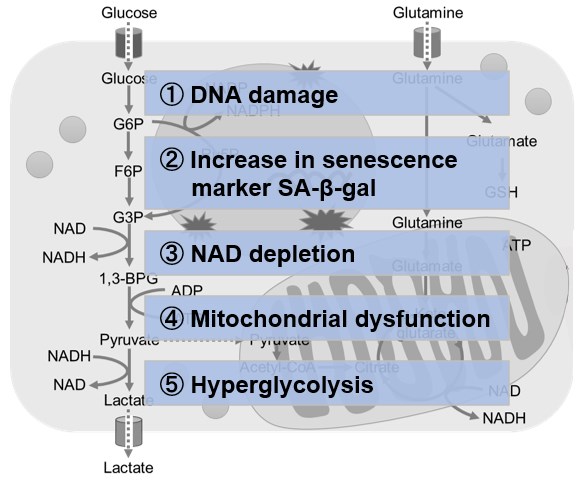
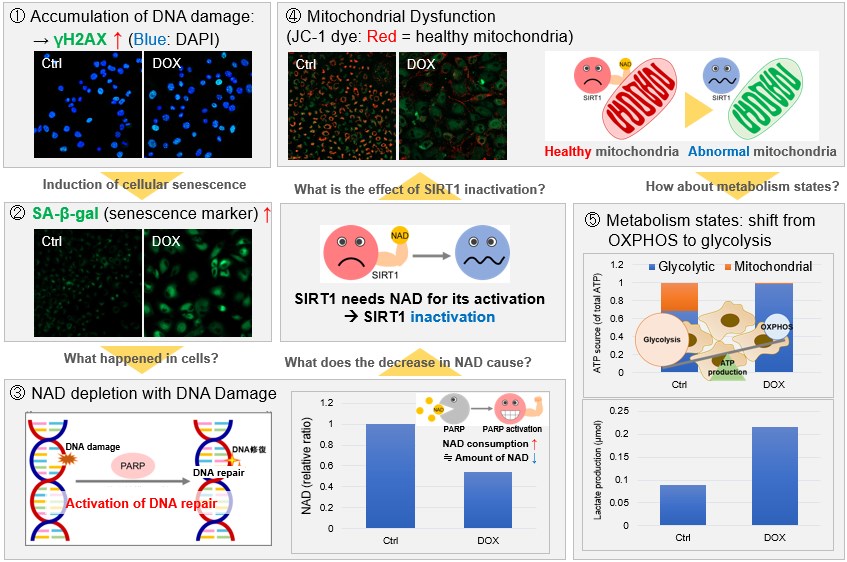
NAD(+) levels decline during the aging process, causing defects in nuclear and mitochondrial functions and resulting in many age-associated pathologies*. Here, we try to redemonstrate this phenomenon in the doxorubicin (DOX)-induced cellular senescence model with a comprehensive analysis of our products. *S. Imai, et al., Trends Cell Biol, 2014, 24, 464-471
|
 |
|