|
In recent years, significant advancements have been made in the understanding of novel exo/endocytic pathways in metabolism. These discoveries have garnered considerable attention, particularly in the field of oncology. For instance, tumor-derived extracellular vesicles have been identified as critical mediators in cancer-induced hepatic reprogramming. Their role, along with TNF inhibition, offers a targetable pathway for both preventing fatty liver formation and enhancing the efficacy of chemotherapy. Another groundbreaking observation reveals that the ingestion of tumor-derived microparticles by macrophages induces a rapid metabolic and phenotypic switch. This change subsequently results in decreased motility in the early stages of metastasis in the lung. Additionally, research has demonstrated that phospholipase D6, a protein found on the mitochondrial outer membrane, accelerates the transport of LDL-LDLR from endocytic vesicles to mitochondria, thereby supporting steroidogenesis.
|
-
Tumour extracellular vesicles and particles induce liver metabolic dysfunction
Click here for the original article: Gang Wang, et al., Nature, 2023
Point of Interest
- All subpopulations of tumor-derived extracellular vesicles and particles (EVPs) could dysregulate liver function.
- The fatty acid cargo of tumor EVPs induced secretion of tumor necrosis factor (TNF) by Kupffer cells, promoting fatty liver formation.
- Kupffer cell ablation or TNF blockade significantly reduced tumor-induced fatty liver formation.
-
Uptake of tumor-derived microparticles induces metabolic reprogramming of macrophages in the early metastatic lung
Click here for the original article: Kelly Kersten, et al., Cell Reports, 2023
Point of Interest
- Ingestion of tumor-derived material leads to the phenotypic reprogramming of macrophages.
- The reprogramming of macrophages influences their patrolling behavior in response to tumor cells.
- ZsGreen+ macrophages demonstrate elevated mitochondrial metabolism, characterized by oxidative phosphorylation (OXPHOS).
- mTORC1 is essential for enhanced oxidative phosphorylation (OXPHOS) and ATP production in ZsGreen+ macrophages.
-
Delivery of low-density lipoprotein from endocytic carriers to mitochondria supports steroidogenesis
Click here for the original article: Yu-Xia Zhou, et al., Nature Cell Biology, 2023
Point of Interest
- PLD6 promotes the entrance of LDL and LDLR into the mitochondria.
- The fusogenic lipid phosphatidic acid generated by PLD6 facilitates the membrane fusion of LDLR vesicles with the mitochondria.
- This intracellular transport pathway of LDL–LDLR bypasses the lysosomes and delivers cholesterol to the mitochondria for steroidogenesis.
|
|
Related Techniques
|
- Endocytosis Detection detection
- ECGreen-Endocytosis Detection
|
|
|
- Lysosomal function
- Lysosomal Acidic pH Detection Kit -Green/Red and Green/Deep Red
|
- Exosome Labeling
- ExoSparkler Exosome Membrane Labeling Kit-Green / Red / Deep Red
-
|
- Plasma Membrane Staining
- PlasMem Bright Green / Red
|
- Lipid droplets detection
- Lipi-Blue / Green / Red / Deep Red
|
- Fatty acid uptake assay
- Fatty Acid Uptake Assay Kit
|
- Oxygen consumption rate assay
- Extracellular OCR Plate Assay Kit
|
|
Related Applications
|
Visualization of EVs uptake via endocytic pathway
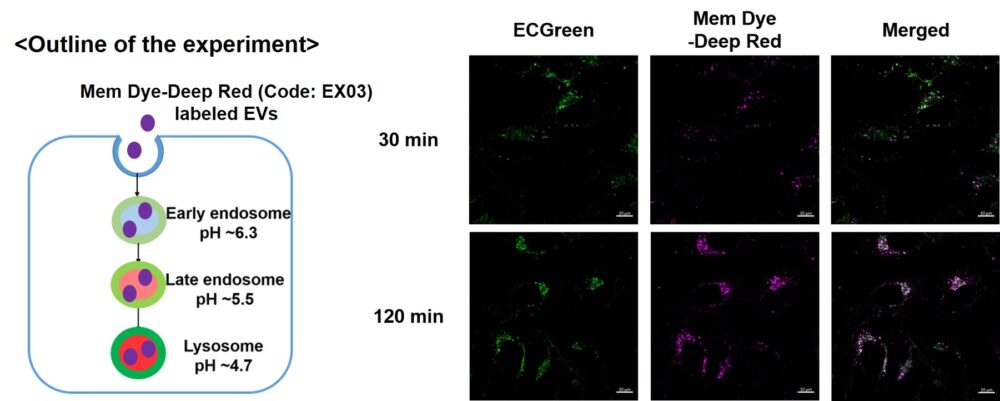
Mem Dye-labeled EVs are internalized via endocytosis:
HeLa cells were incubated with 10 μmol/L ECGreen for 30 min. Then, Mem Dye-Deep Red labeled EVs (quantified as 10 µg of protein) were added to HeLa cells. After 30 or 120 min incubation, the cells were washed and observed under a fluorescence microscope (Scale Bar: 10 µm).
Observation of temperature-dependent endocytosis changes in floating cells
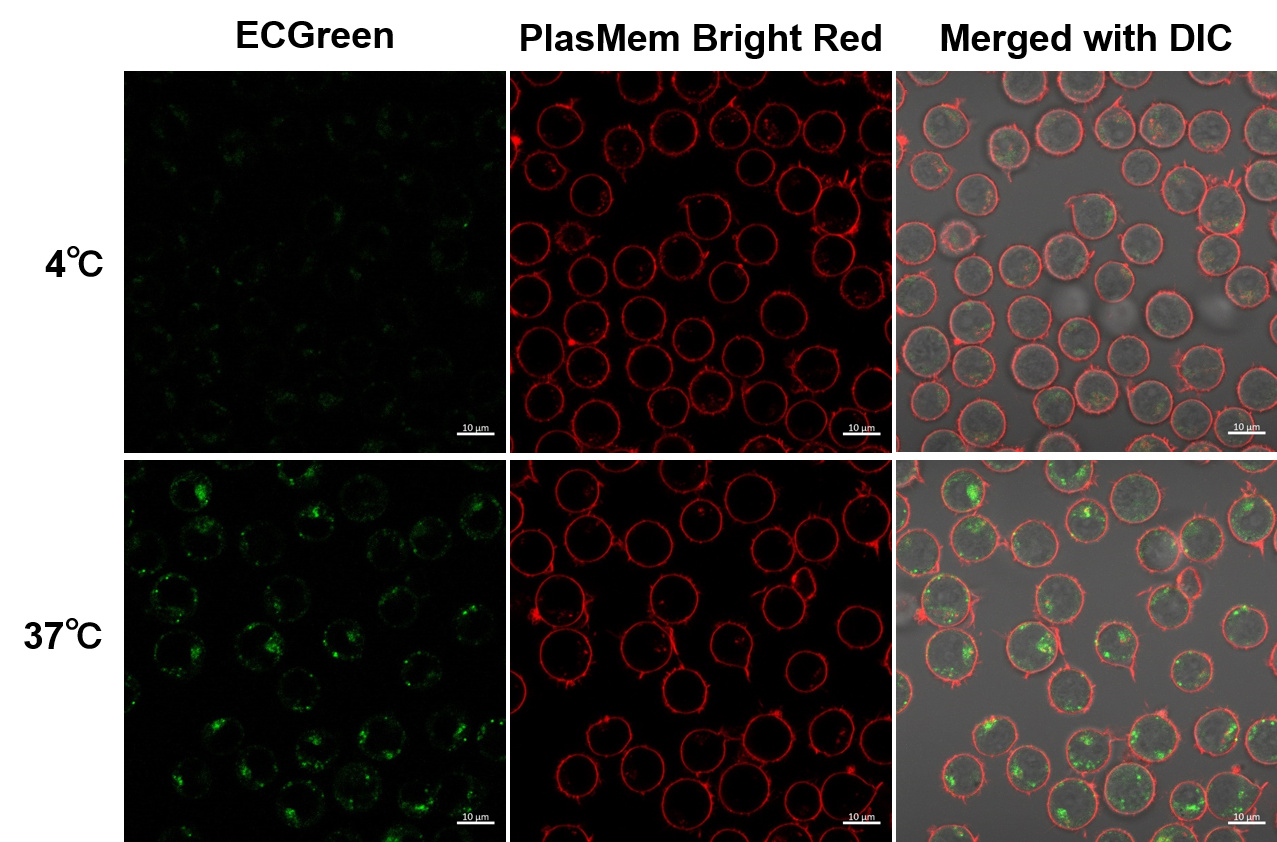
Temperature-dependent changes in endocytosis of Jurkat cells were visualized using ECGreen-Endocytosis Detection and PlasMem Bright Red. Cold incubation inhibits the endocytic pathway as observed with ECGreen and PlasMem Bright red.
|

















