|
Exosomes, a form of extracellular vesicles (EVs), mediate intercellular communication by exchanging proteins, DNA, RNA, and lipids between donor and recipient cells, and modulate the biological activities in target cells through receptor-ligand interactions. Recent findings suggest that EV-mediated communications contribute to malignant transformation and the metastasis of cancer, and several disease progressions. Consequently, EVs are attracting considerable interest in the scientific community. Today, we introduce you to three highlighted articles focusing on EVs-mediated communications related to diabetes, immunosystem, and aging.
|
-
Extracellular vesicles mediate the communication of adipose tissue with brain and promote cognitive impairment associated with insulin resistance
Click here for the original article: Jin Wang, et al., Cell Metabolism, 34, 1264-1279, 2022
Point of Interest
- EVs and their cargo miRNAs mediate adipose tissue-brain inter-organ communication are used in this study - Adipose tissue-derived EVs induce cognitive impairment associated with insulin resistance
- miR-9-3p in adipose tissue-derived EVs mediates synaptic damage
- Targeting adipose tissue-derived EVs or miRNAs prevents cognitive impairment in diabetes
-
An intercellular transfer of telomeres rescues T cells from senescence and promotes long-term immunological memory
Click here for the original article: Alessio Lanna, et al., Nature Cell Biology, 24, 1461-1474, 2022
Point of Interest
- The common view is that T lymphocytes activate telomerase to delay senescence
- Upon contact with T cells, antigen-presenting cells (APCs) degraded shelterin to donate telomeres and then transferred in EVs at the immunological synapse
- Antigen-specific populations of T cells decide aging fate, and it is based on telomere vesicle transfer upon initial contact with APCs
- These telomere-acquiring T cells are protected from senescence, conferring long-lasting immune protection
-
Small extracellular vesicles from young adipose-derived stem cells prevent frailty, improve health span, and decrease epigenetic age in old mice
Click here for the original article: Jorge Sanz-Ros, et al., Science Advances, 8, eabq22, 2022
Point of Interest
-- Small EVs (sEVs) derived from adipose mesenchymal stem cells (ADSCs) of young animals brought an improvement in several parameters usually altered with aging
- ADSC-sEVs induced pro-regenerative effects and a decrease in oxidative stress, inflammation, and senescence markers in muscle and kidney
- Predicted epigenetic age was lower in tissues of old mice treated with ADSC-sEVs and their metabolome changed to a youth-like pattern
- The microRNAs contained in sEVs might be responsible for the observed effects
|
|
Related Techniques
|
- Endocytosis Detection detection
- ECGreen-Endocytosis Detection
|
|
|
- Lysosomal function
- Lysosomal Acidic pH Detection Kit -Green/Red and Green/Deep Red
|
- EVs Labeling
- ExoSparkler Exosome Membrane Labeling Kit-Green / Red / Deep Red
|
- EVs Isolation
- ExoIsolator Exosome Isolation Kit
|
- Cellular senescence detection
- SPiDER-βGal for live-cell imaging or flow cytometry / microplate reader / tissue samples
SPiDER-βGal Blue for fixed cell and for multiple staining with immunostaining and other methods
|
- Plasma Membrane Staining
- PlasMem Bright Green / Red
|
- Lipid droplets detection
- Lipi-Blue / Green / Red / Deep Red
|
- Fatty acid uptake assay
- Fatty Acid Uptake Assay Kit
|
- Oxygen consumption rate assay
- Extracellular OCR Plate Assay Kit
|
|
Related Applications
|
Visualization of EVs uptake via endocytic pathway
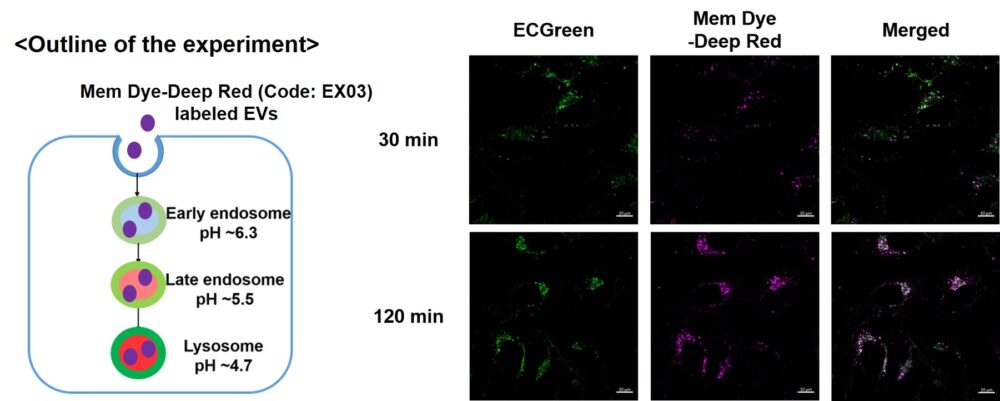
Mem Dye-labeled EVs are internalized via endocytosis:
HeLa cells were incubated with 10 μmol/L ECGreen for 30 min. Then, Mem Dye-Deep Red labeled EVs (quantified as 10 µg of protein) were added to HeLa cells. After 30 or 120 min incubation, the cells were washed and observed under a fluorescence microscope (Scale Bar: 10 µm).
Observation of temperature-dependent endocytosis changes in floating cells
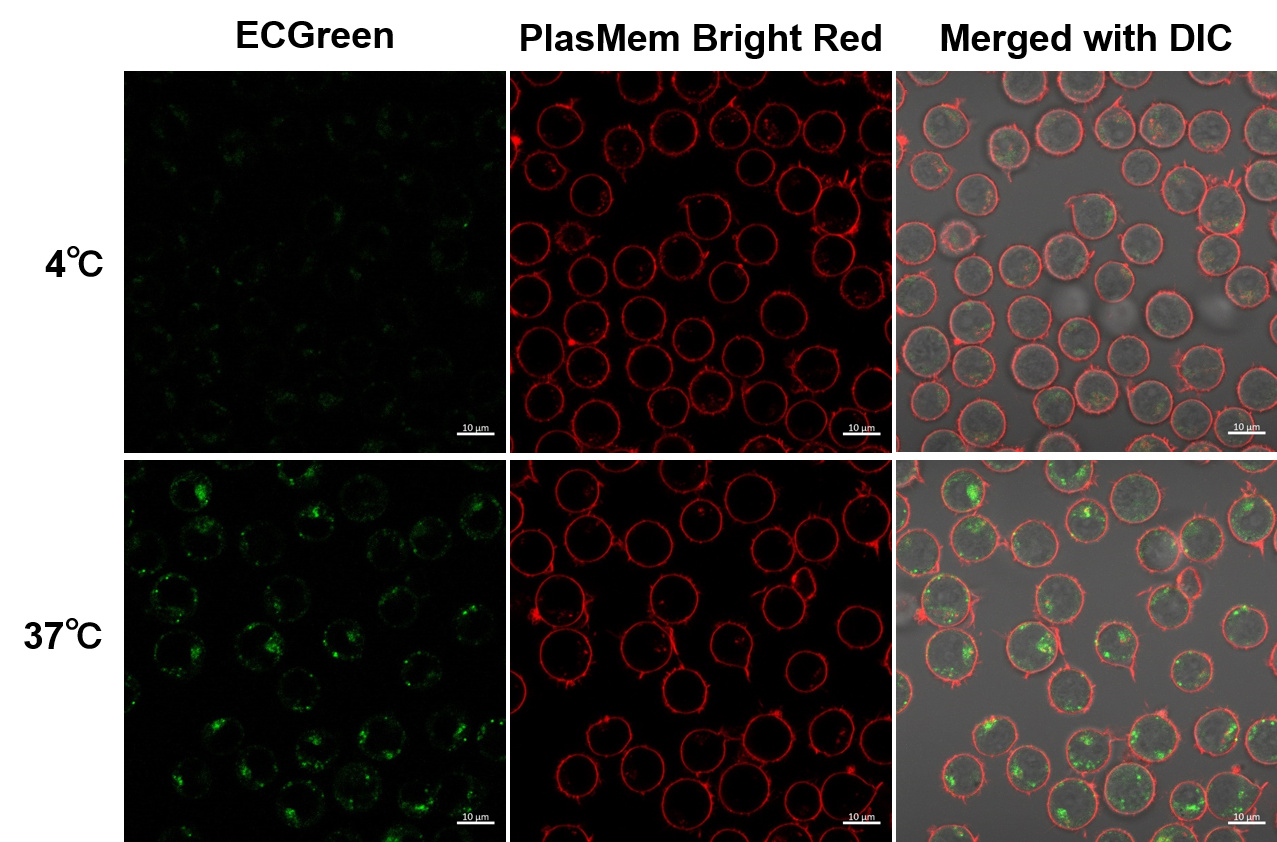
Temperature-dependent changes in endocytosis of Jurkat cells were visualized using ECGreen-Endocytosis Detection and PlasMem Bright Red. Cold incubation inhibits the endocytic pathway as observed with ECGreen and PlasMem Bright red.
|

















