|
Metabolic reprogramming is a characteristic feature of cancer, through which changes in energy production and biosynthetic pathways enable cancer cells to adapt to their environment and support proliferation, metastasis, and tumor growth. Recent studies show that cancer metabolism is regulated not only by cell intrinsic changes but also by interactions with the tumor microenvironment. Caveolin-2 expressed in tumor associated nerves induces a shift from glycolysis to mitochondrial metabolism in head and neck squamous cell carcinoma, supporting tumor initiating capacity and therapy resistance. In human glioblastoma, glucose derived carbon is preferentially directed toward nucleotide and NAD biosynthesis rather than the TCA cycle, distinguishing tumor tissue from the normal cortex. Together, these findings highlight diverse modes of metabolic reprogramming in cancer. |
||||||||||||||||||||||
|
CAV2-expressing nerves induce metabolic switch toward mitochondrial oxidative phosphorylation to promote cancer stemness (Nature Communications, 2025) Highlighted technique: In this study, to examine how tumor-associated nerves composed of CAV2-expressing neural and glial cells affect the metabolic state of head and neck squamous cell carcinoma cells, cancer cells were analyzed under co-culture conditions with CAV2-expressing nerves. Glycolytic dependence was assessed by lactate production, while mitochondrial oxidative metabolism was evaluated by oxygen consumption rate (OCR) analysis, supported by multiple complementary assessments of mitochondrial function and content |
||||||||||||||||||||||
|
Rewiring of cortical glucose metabolism fuels human brain cancer growth (Nature, 2025) Highlighted technique: To compare how glucose-derived carbon is utilized in glioblastoma and the normal cortex in the human brain, glioblastoma patients received intravenous 13C-labeled glucose. Resected glioblastoma and matched cortical tissues were analyzed by LC–MS to quantify isotope incorporation into glycolytic intermediates, TCA cycle metabolites, neurotransmitter-related metabolites, nucleotides, and NAD, and 13C-based metabolic flux analysis was used to comprehensively assess the metabolic fate of glucose-derived carbon. |
||||||||||||||||||||||
Metabolic Activity Assays (click to open/close)
|
||||||||||||||||||||||
Application Note (click to open/close)
|
||||||||||||||||||||||
|
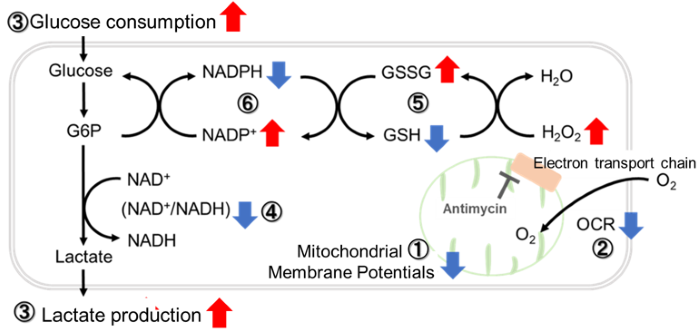
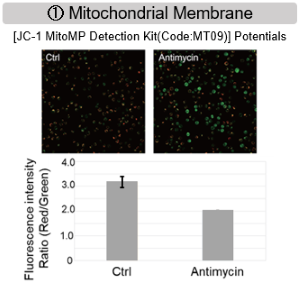
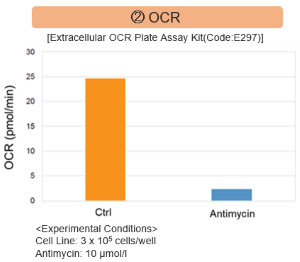
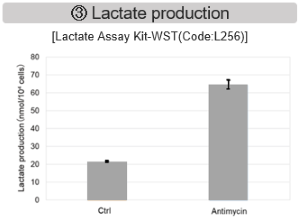
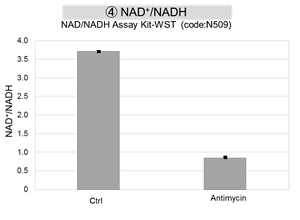
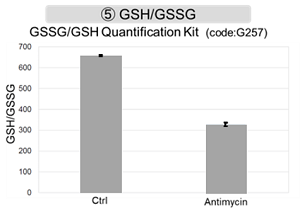
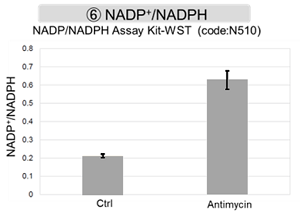
Antimycin stimulation of Jurkat cells was used to evaluate the changes in cellular state upon inhibition of the mitochondrial electron transport chain using a variety of indicators.
|
The results showed that inhibition of the electron transport chain resulted in (1) a decrease in mitochondrial membrane potential and (2) a decrease in OCR. In addition, (3) the NAD+/NADH ratio of the entire glycolytic pathway decreased due to increased metabolism of pyruvate to lactate to maintain the glycolytic pathway, (4) GSH depletion due to increased reactive oxygen species (ROS), and (6) increase in the NADP+/NADPH ratio due to decreased NADPH required for glutathione biosynthesis were observed. Products in Use |
|
|
|