|
Disrupted neuronal energy metabolism drives neurodegeneration by triggering cascades that lead to synaptic failure, neuronal loss, and clinical decline. Neurons primarily rely on OXPHOS. When OXPHOS is limited by hypoxia or mitochondrial dysfunction, they increase reliance on glycolysis. However, excessive glycolysis is harmful because toxic by-products accumulate. In Purkinje cells, ATG5-dependent autophagy limits GLUT2 and restrains glucose uptake, preventing glycolytic overdrive and supporting survival. In another study, neurons under stress mobilize endogenous glycogen to boost glycolysis as a metabolic backup and preserve function. Together, these findings show that neurons adjust their glycolytic reliance in response to environmental constraints. |
||||||||||||||||
|
Autophagy regulator ATG5 preserves cerebellar function by safeguarding its glycolytic activity (Nature Metabolism, 2025) Related techniques Glucose Uptake Assay, Autophagy Detection |
||||||||||||||||
|
Glycogen supports glycolytic plasticity in neurons (PNAS, 2025) Highlighted technique: In this study HYlight, a gene-encoded ratiometric fluorescent sensor for the glycolytic intermediate fructose-1,6-bisphosphate, was used to visualize cellular glycolytic activity in real time. The sensor combines an FBP-binding domain with a modified cpGFP, is excited at 405 and 488 nm, and upon FBP binding the two signals shift in opposite directions so changes in their ratio report increases or decreases in glycolysis. Related techniques Glycolysis/OXPHOS Assay, Mitochondrial Membrane Potential |
||||||||||||||||
Metabolic Activity Assays (click to open/close)
|
||||||||||||||||
Application Note (click to open/close)
|
||||||||||||||||
|
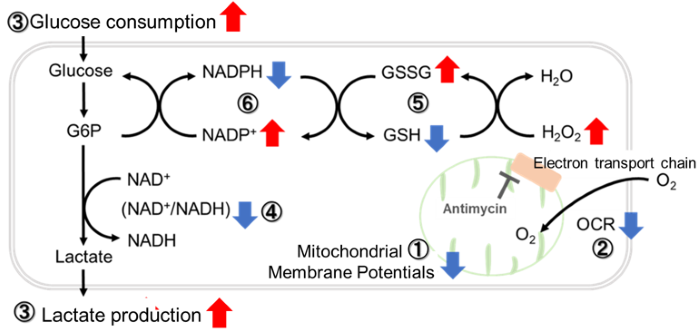
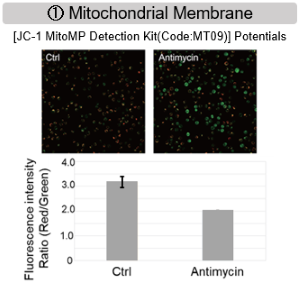
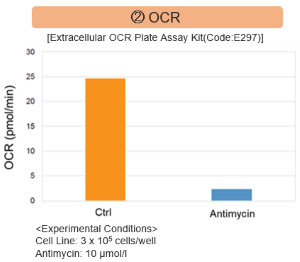
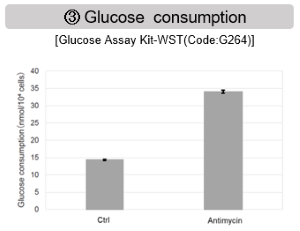
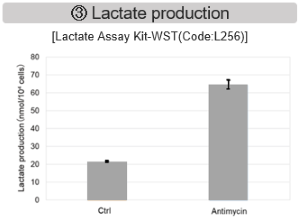
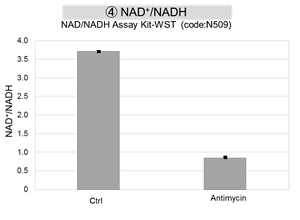
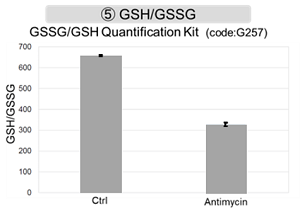
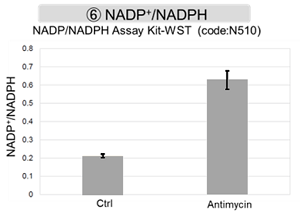
Antimycin stimulation of Jurkat cells was used to evaluate the changes in cellular state upon inhibition of the mitochondrial electron transport chain using a variety of indicators.
|
The results showed that inhibition of the electron transport chain resulted in (1) a decrease in mitochondrial membrane potential and (2) a decrease in OCR. In addition, (3) the NAD+/NADH ratio of the entire glycolytic pathway decreased due to increased metabolism of pyruvate to lactate to maintain the glycolytic pathway, (4) GSH depletion due to increased reactive oxygen species (ROS), and (6) increase in the NADP+/NADPH ratio due to decreased NADPH required for glutathione biosynthesis were observed. Products in Use |
|
|
|