|
In recent years, the discovery of several novel lipid-mitochondria axis in metabolism has attracted much attention. A link between acylcarnitine accumulation and lipid intolerance in skeletal muscle mitochondria is tightly linked to poor health. In other breakthroughs, decreased expression in fatty acid transporters and β-oxidation enzymes causes fatty acid buildup in lipid droplets. Research also uncovers that knocking out a vital enzyme in mitochondrial fatty acid oxidation astrocytes leads to cognitive impairment.
|
-
Pyruvate-supported flux through medium-chain ketothiolase promotes mitochondrial lipid tolerance in cardiac and skeletal muscles
Click here for the original article: Timothy R. Koves, et. al., Cell Metabolism, 2023.
Point of Interest
- The metabolic pathway of long-chain fatty acid oxidation is susceptible to bottlenecks.
- Bottlenecks in the oxidation of fat can lead to CoA trapping and a decrease in respiratory efficiency.
- The reverse flux of pyruvate-derived acetyl CoA through the medium-chain ketothiolase pathway can regenerate free CoA.
- High levels of medium-chain ketothiolase in the heart and red muscles enhance mitochondrial tolerance to lipids.
-
Arf1 coordinates fatty acid metabolism and mitochondrial homeostasis
Click here for the original article: Ludovic Enkler, et. al., Nature Cell Biology, 2023.
Point of Interest
- Expression of fatty acid transporters and the key enzyme in β-oxidation decreased in cells with a hyperactive Arf1 mutant, causing fatty acids to accumulate in lipid droplets.
- As a result, mitochondria fragmented and ATP synthesis declined.
- Genetic and drug-induced reduction of fatty acids mimicked the mitochondrial effects of the arf1 mutant.
- Although β-oxidation occurs in both mitochondria and peroxisomes in mammals, Arf1's role in fatty acid metabolism remains conserved.
-
Fatty acid oxidation organizes mitochondrial supercomplexes to sustain astrocytic ROS and cognition
Click here for the original article: Brenda Morant-Ferrando , et. al., Nature Metabolism, 2023.
Point of Interest
- Knockout of CPT1A, a key enzyme of mitochondrial fatty acid oxidation, leads to cognitive impairment in adult mouse astrocytes.
- Decreased fatty acid oxidation alters astrocytic pyruvate metabolism and reduces reactive oxygen species formation.
- Astrocytes naturally metabolize fatty acids to maintain the mitochondrial respiratory chain in an energetically inefficient, disassembled conformation.
- This disassembled conformation helps in preserving the signaling of reactive oxygen species and supports cognitive performance.
|
| Related Techniques |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Related Applications |
<Fatty acid starvation induced by uptake inhibitor evoke reprogramming of cellular metabolism >
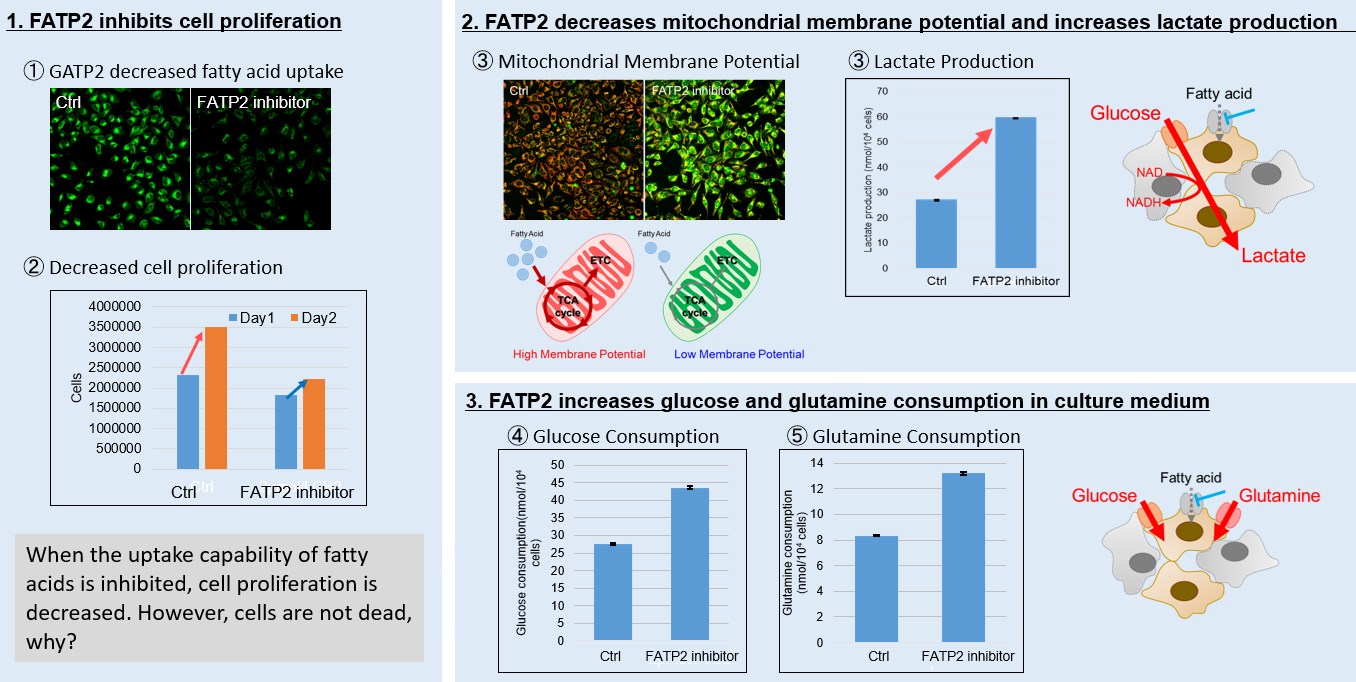
|
Mitochondrial fatty acid β-oxidation and oxidative phosphorylation (OXPHOS) are crucial biochemical processes that metabolize fats and sugars to produce ATP, the cell's primary energy source. In this section, we underscored the significance of fatty acid starvation and energy pathways, with an emphasis on the fatty acid uptake inhibitor, FATP2. Here are the key findings from our experiments conducted on HeLa cells:
・Inhibition of fatty acid uptake results in reduced cell proliferation, though it does not lead to cell death. This was determined through the use of a Cell Counting Kit-8 and Fatty Acid Uptake Kit (Image 1).
・Fatty acid starvation shifts cellular metabolism from OXPHOS to glycolysis, as indicated by the Glycolysis/JC-1 MitoMP Assay Kit. (Image 2)
・When fatty acid uptake is inhibited, a compensatory increase in glucose and glutamine uptake occurs to preserve cell viability, as observed using the Glucose Assay Kit and Glutamine Assay Kit. (Image 3)
|
















